Competency C2: Install Residential Water Treatment Systems
Learning Task 2
Describe the Sizing of Water Treatment Equipment
Learning objectives
After completing the learning tasks in this chapter, you will be able to:
- describe the processes involved in testing the water supply
- identify water contaminants and their acceptable levels, and
- select methods for the treatment of contaminants in a water supply.
A surface or shallow well supply is more prone to turbidity and bacterial contamination than is water from a deep well source, while a deep well supply is more prone to contamination from iron, hardness, and gases than is a ground source. Regardless, a residential water supply that is not from a community system must be checked for both chemical and bacterial contamination, followed by the prescription of appropriate remedies if found. This is done by drawing and testing a water sample.
Testing the Water
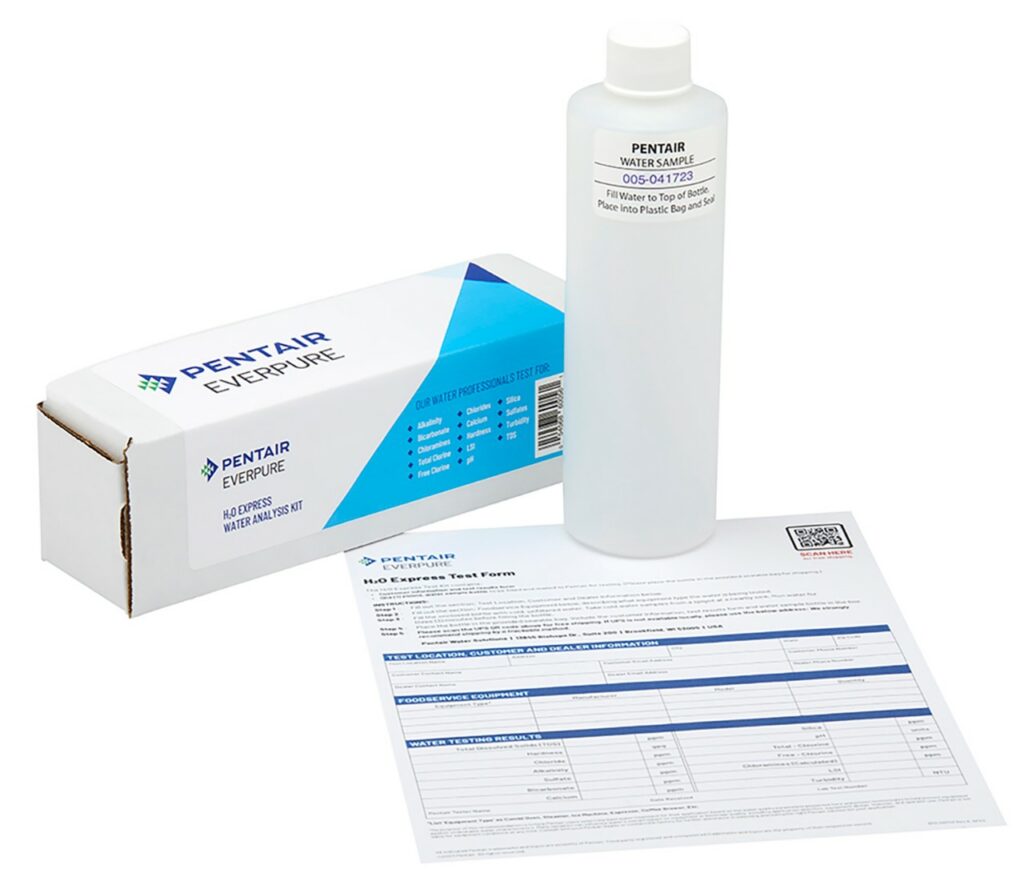
Water samples should be taken as near the source as possible. This can be either from the well itself, or from a faucet as close to the well as possible. A glass or plastic container should be used and first cleaned and rinsed with the test water, which ideally should be between 20 - 25°C (68 - 77°F). The collected sample can either be tested on-site or be sent to a facility operated by a water treatment equipment manufacturer such as Novo® (formerly Watergroup®). Although taking longer to obtain, test results from a facility are usually more comprehensive and accurate, and don’t require the sampler to purchase expensive test kits. As well, facility testing is usually manufacturer-specific, so the facility often suggests appropriate treatment equipment for the samples’ results, in the hope that their equipment will also be chosen. A more thorough laboratory analysis can be performed on request if contamination by industrial wastes is suspected.
On-site testing can be very accurate but is limited to the scope of test equipment available and the knowledgeable practices of the tester. Note that both onsite and facility testing are limited to testing for chemical contamination only. Bacterial testing must be done by sending a water sample in a sterilized container to a government-approved laboratory, which can also test for iron bacteria. Iron bacteria cannot be detected by a standard iron test. Local health units, as well as plumbing wholesalers, normally carry water sample bottles and laboratory request forms for both types of tests. Tests for bacteriological content should be done on a regular basis (at least annually), as this aspect of a water source can change without creating a noticeable change in water quality.
A basic chemical analysis will reveal hardness and iron concentrations as well as the presence of manganese, total dissolved solids (TDS) and pH levels. Tests for hydrogen sulphide (H2S) are most accurate if performed onsite but can be done in a facility provided the test is performed within a short time of the sample being drawn, usually 24 hours, as H2S concentrations rapidly diminish after being exposed to air. While the presence of any of the above contaminants causes concern in various ways, they are usually considered an aesthetic issue and the choice to correct any water problems they cause lies with the homeowner.
A bacterial test will reveal the possible presence of two types of bacteria, which are:
- Total coliforms – these occur naturally in soil and in the guts of humans and animals, and their presence may indicate fecal contamination.
- Escherichia coli (E. coli) – these originate only in the guts of humans and animals, and their presence indicates definite fecal (sewage) pollution.
Although the 1996 edition of Health Canada’s Guidelines for Canadian Drinking Water Quality (6th edition) indicates that no more than 10 total coliform bacteria per 100 ml in water is marginally safe to drink, the most recent September 2022 edition indicates that 0 total coliform per 100 ml of water is now the standard. Both editions indicate that water containing any measured amount of E. coli is unsafe to drink and that corrective action should be taken immediately.
Test Equipment and Procedures
Basic onsite water testing may be performed using test kits provided by water treatment equipment suppliers. Test kits can contain reagents, buffering compounds and colour comparison components, and can be single-task (checking for one water condition only) or multiple-task (checking for two or more conditions) as desired. Hardness, iron, manganese, pH, and chlorine content can all be detected onsite using chemical reagents. The only basic test that cannot be completed using chemicals is a measurement of total dissolved solids (TDS). This involves using a small electronic scale that measures the electrical conductivity of water, indicating the concentration of conductive metals in the water.

The chemicals in the test kits are age-sensitive and should be used within the expiry dates published. Any tests performed with out-of-date reagents should be considered inaccurate. Freezing or high temperatures may also affect these chemicals, and exposure to such conditions should be avoided.
Testing for Hardness
The simplest test for the presence of hardness minerals (calcium and magnesium) involves no test kit. This is called a soap test and involves taking a 5 to 20 ml sample of the water to be tested, in a 50 to 100 ml clean, clear container and adding 2 or 3 drops of dishwashing liquid to it. Cover the container and shake it vigorously for a few seconds. If this action produces suds, the water is relatively soft. If there are few suds but the water appears to be cloudy and has curds or lumps, the water is hard.
Hach® is a well-known manufacturer of water contaminant test kits, and the procedures that follow are particular to their products. We will limit the explanation of a chemical test procedure to this one for hardness. Other procedures, such as those for iron, manganese, etc. will be similar in execution but will involve different reagents and amounts. Always follow the instructions contained within any test kit carefully.
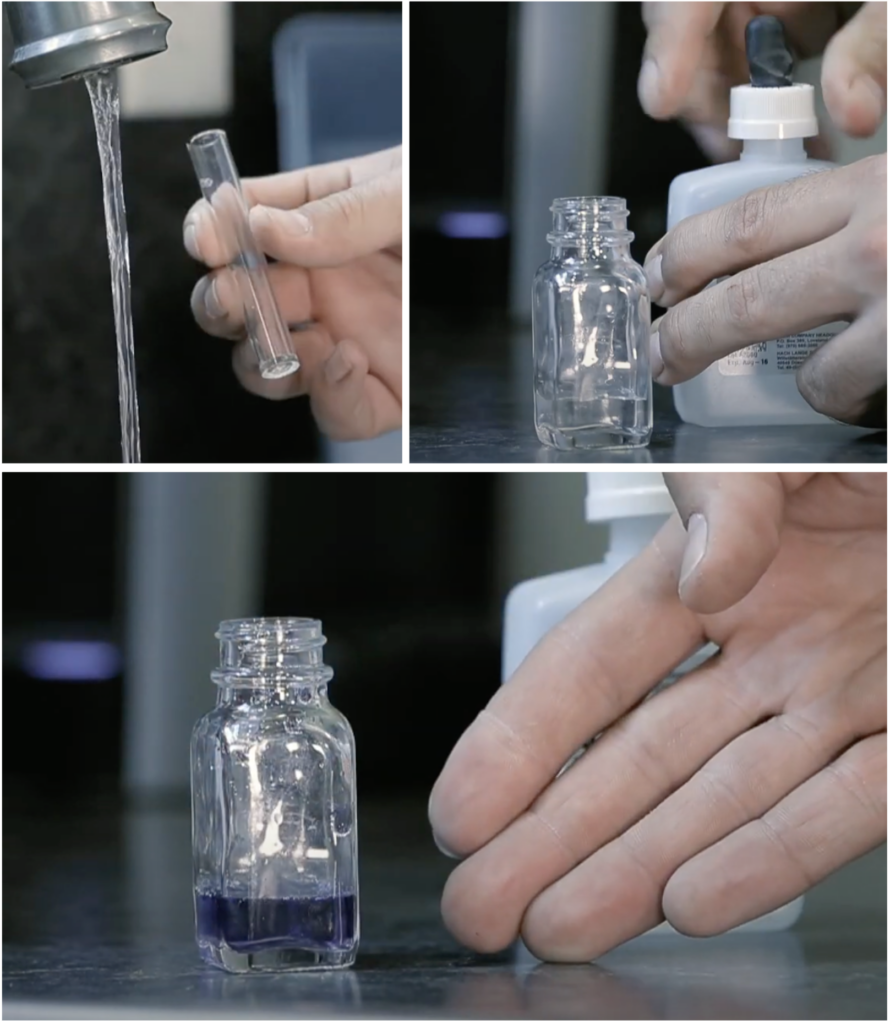
To test for hardness using a Hach® test kit, fill the plastic measuring tube to its fullest level with the water to be tested, and then pour it into the glass mixing bottle. Next, add the contents of the hardness reagent “UniVer 3” contained within a plastic pillow-type container. Swirl the mixing bottle to mix the reagent with the water sample. A blue colouration indicates soft water, and nothing further is required.
If the colour is red, hard water is indicated. Drop by drop, a titrant agent “Hardness 3” is added, with the mixing container swirled after each drop and the number of drops being counted. Continue adding titrant drops until the colour changes from red to blue. Each drop of titrant represents 1 grain per gallon hardness. This procedure is used for hardness up to 30 grains per gallon (gpg). If the number of drops reaches 30, with no change in colour, a similar procedure involving a different reagent must be used.
Testing for Iron
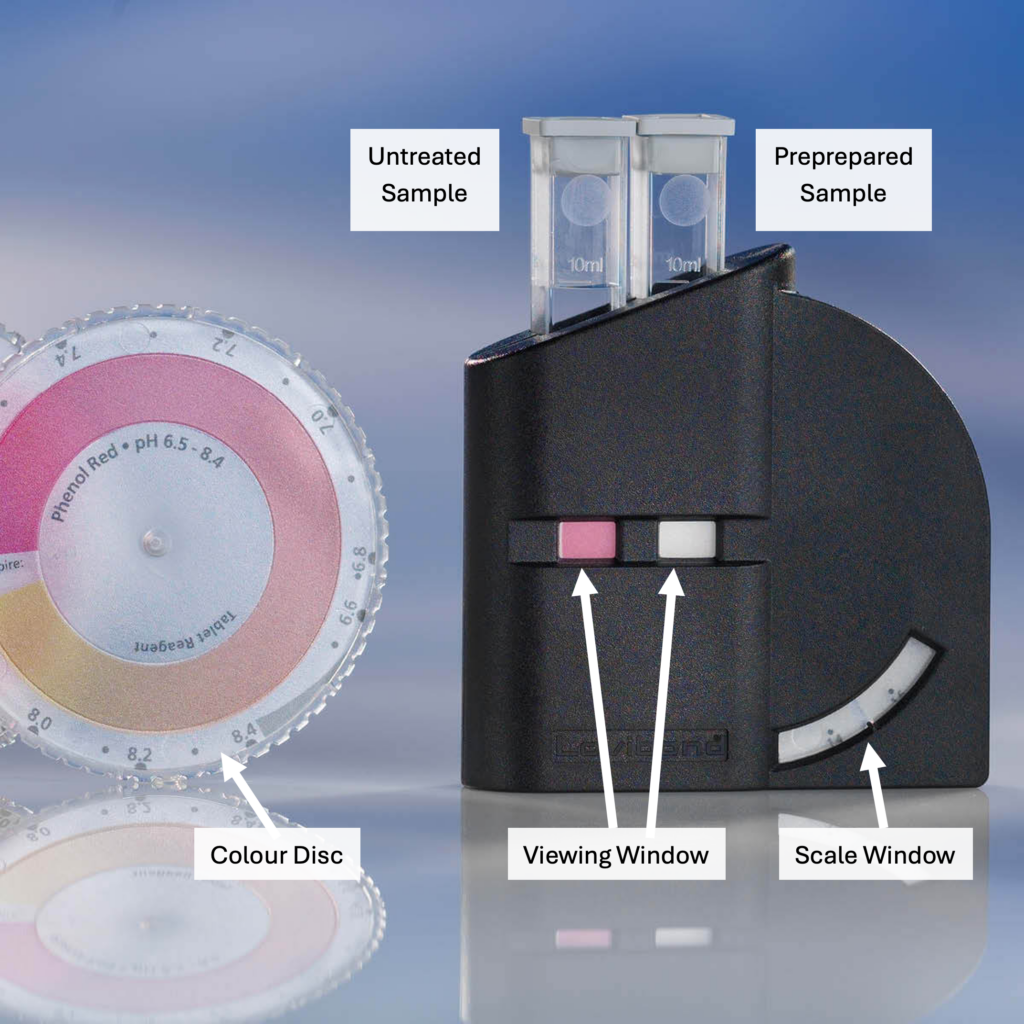
A different test kit, specific to iron in water, is used whereby, like the hardness test, a specified amount of reagent added to a measured amount of water causes a colour change in the sample. The sample’s colour is compared side-by-side to a fixed colour comparison strip or wheel that has different shades of orange, indicating different levels of iron. Iron tests must be completed within a very short time of the sample being drawn, as exposure to air (oxygen) will alter test results quite quickly.

Testing for Manganese and pH
The preparation of a water sample is similar to that for iron in water, using a specific test kit for manganese and a different one for pH. Procedures for manganese and pH tests will be similar as well, involving a colour comparison against a fixed chart while using differing amounts of reagents and volumes of sample water.
Testing for TDS
As mentioned and shown earlier, testing for total dissolved solids doesn’t use any reagents to cause a colour change. Rather, a specially designed meter is used where a small current, produced by batteries, is pushed through the water sample. The presence of dissolved metals causes water to be more electrically conductive, and consequently the higher the concentration of TDS, the more conductive the water will be. This is measured on a scale in parts per million (ppm).
Interpreting the Results
Hardness
A test result for hardness is normally expressed in grains per gallon of calcium carbonate (CaCO3) equivalent but can also be expressed as parts per million. Because there are both US and Imperial gallons in a water professional’s vocabulary, there are two conversions at play. As mentioned earlier, there are 7000 grains per pound. Therefore, if one US gallon of water weighs 8.33 pounds, the conversion of gpg to ppm is:
1 gpg (US) = [latex]\Large \frac{\frac{1}{7000} \text{lb.}}{8.33 \text{lb.}} \times 1\,000\,000[/latex] = 17.1 ppm per 1 US gallon
The conversion between gpg and Imperial gallons will be:
1 gpg (Imp) = [latex]\Large \frac{\frac{1}{7000} \text{ lb.}}{10 \text{ lb.}} \times 1\,000\,000[/latex] = 14.3 ppm per 1 Imp gallon
Remembering also that ppm and mg/l are equivalent measures, the following classifications of hardness in water can be assumed. These are taken from the November 2022 edition of “Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Hardness”:
- 0 to < 60 mg/L……..… soft
- 60 to < 120 mg/L…..… medium hard
- 120 to < 180 mg/L..…. hard
- 180 mg/L and above.... very hard
Equipment manufacturers may publish limits that differ from those above and should be considered as applicable to their equipment only. As well, the above terms such as “soft” and “hard” are for reference only - when choosing equipment to treat for hardness, the actual measurements in mg/l, ppm, or gpg are used. These measurements are known as “total hardness”, meaning a summary of all the chemical constituents in the water. Total hardness of water is made up of “temporary hardness” and “permanent hardness” in varying proportions. Temporary and permanent hardness are terms used to denote bicarbonates (temporary) and carbonates (permanent) of calcium and magnesium dissolved in water. Water heated to over 140°F (60°C) in a vessel, such as a hot water tank, will cause bicarbonates to settle out and attach to surfaces as scale deposits. These deposits will clog piping and pipe outlets, causing reduced flow and efficiency issues. Water heated to temperatures exceeding 299°F (148°C), such as occurs in steam heating boilers, will cause carbonates, sulphates, nitrates, and chlorides to settle out. These constituents will quickly form a sludge layer in the bottoms of the pressure vessels and are usually removed daily by quickly opening a low-point outlet and using the steam’s pressure to flush the sediment (“blow down”) to a safe location.
Hard water causes incrustation in distribution systems and excessive soap consumption, whereas soft water may result in corrosion of metal water pipes. Also, public acceptability of the degree of hardness may vary considerably from community to community depending on local conditions. Therefore, a maximum acceptable level for hardness cannot be specified, and the following conditions are assumed (from “Guidelines for Canadian Drinking Water Quality”:
- hardness levels between 80 and 100 mg/L (as CaCO3) are generally considered to provide an acceptable balance between corrosion and incrustation.
- waters with hardness levels in excess of 200 mg/L are considered poor but have been tolerated by consumers, and
- waters with hardness in excess of 500 mg/L are unacceptable for most domestic purposes.
It is generally recommended that hardness in household applications not exceed 60 mg/l (3.5 US gpg or 4 Imp gpg). The use of a water softener is recommended when total hardness exceeds these limits but will only be effective if not over 100 gpg.
Iron
Iron is an essential element in human nutrition and intake of iron from a typical Canadian diet is more than sufficient to meet the minimum daily requirement. Toxic effects have resulted from the ingestion of large quantities of iron, but there is no evidence to indicate that concentrations of iron commonly present in food or drinking water constitute any hazard to human health. Therefore, a maximum acceptable concentration has not been set. At concentrations above 0.3 mg/L, iron can stain laundry and plumbing fixtures and produce undesirable tastes in beverages. The precipitation of excessive iron imparts an objectionable reddish-brown colour to water. Iron may also promote the growth of certain micro-organisms, leading to the deposition of a slimy coating in water distribution pipes (iron bacteria).
Generally, only a small percentage of the population will be able to taste iron in drinking water at concentrations below 0.3 mg/L, so the aesthetic objective for iron in drinking water is therefore ≤0.3 mg/L. Water softeners are capable of removing iron within these limits, up to 3 mg/l. However, ferric iron tends to foul the softener bed, so the backwashing and rinsing processes must be very thorough. The addition of a resin conditioner to the brine tank is recommended to assist in keeping the resin bed operational. At levels over 3 mg/l, a stand-alone iron filter is recommended. These are similar to a softener but have a filter bed of natural or synthetic manganese greensand and use a potassium permanganate solution as the regenerating agent rather than salt. Depending on the manufacturer, these will remove up to 10 mg/l of ferrous and ferric iron but will not remove iron bacteria. Chemical-free iron filters facilitate the removal of ferric and ferrous iron as well as iron bacteria by introducing a small amount of air into the system using a hydrocharger. The air causes oxidation of the iron in a special filter media within the tank, which needs no chemical reaction to be regenerated. This method works well on higher concentrations of iron, whereas the manganese greensand filter is more effective for lower concentrations.
Polyphosphate feeders and chlorinators are also effective for iron removal but require certain specific conditions for their use to be considered.
Manganese
Health Canada has set the Maximum Acceptable Concentration (MAC) of 0.12 mg/L for manganese in drinking water. The MAC is intended to protect all Canadians and is based on the most vulnerable/sensitive population (e.g. infants and young children). Health Canada has also established a new Aesthetic Objective for manganese of 0.02 mg/L. Manganese in water at this concentration is not a health concern, but it may affect the colour or appearance of the water and can cause staining of fixtures and laundry. Water softeners and manganese greensand filters will reduce manganese content to acceptable levels, with manganese greensand filters requiring more care and attention due to resin bed fouling issues.
pH
Regardless of the form of test apparatus used (electronic potentiometers, test sensors, colour discs or test strips), water will need pH correction if it is outside the normally accepted range of pH 7.0 – 10.5 (Health Canada’s “Guidelines for Canadian Drinking Water Quality”). Neutralizing filters are the most common residential correction device used for water that is acidic (pH <7.0), with soda ash feeders normally used for municipal water supplies as they require more careful monitoring. Extremely alkaline waters result from carbonates and bicarbonates in the earth and are not as common an occurrence as acidic water. Some cities in Canada, such as Vancouver, are increasing the target alkalinity levels in their water systems to between pH 8.3 to 8.5 to help control corrosion of copper pipes and inhibit the leaching of lead from soldered connections into drinking water.
TDS
The readings obtained by a conductivity test are expressed in microsiemens per centimetre (uS/cm). This can be multiplied by 0.65 to give an approximate reading for total dissolved solids in mg/l. Through taste testing, Health Canada has determined that an aesthetic objective of ≤500 mg/L has been established for TDS in drinking water. Although not usually a health concern, excessive levels of TDS can decrease the effectiveness of a water softener. In applications where the water is high in TDS, only reverse osmosis, demineralization, or distillation will work to improve water quality.
Calculating Water Softening Demand
The water conditioning industry widely recommends a three-day service period for water softeners, meaning they should be sized to allow soft water to be supplied to a household for three days before requiring regeneration. This allows a reserve capacity and has proven to achieve the most economical operational costs.
The water quality analysis will determine the grains per gallon content of calcium and magnesium, expressed as gpg of calcium carbonate, and will also report a reading of iron and manganese, if present, in ppm or mg/l. In order to size a softener, these three possible contaminants must be expressed as one term, which is gpg of hardness, so conversions must be made. The following are values commonly used by many softener manufacturers:
- every 1 ppm of iron is expressed as 4 gpg of hardness
- every 1 ppm of manganese is expressed as 8 gpg of hardness
It is important to use the values prescribed by the softener manufacturer chosen. These amounts would be added to the gpg hardness figure for calcium carbonate to arrive at a total hardness count for the sizing of the softener.
The sizing of a water softener is a mathematical issue, using the following factors:
- the hardness, iron, and manganese content of the water, expressed in grains per gallon (gpg)
- the number of people in the household using the water
- the average use of each person in gallons per day, and
- a 3-day regeneration period
The total hardness, number of people using the water system, and regeneration period are all easy to establish, whereas the average daily use of water per person can vary. The 2019 document “Statistics Canada, Table 38-10-0271-01 “Potable water use by sector and average daily use” has established that the average daily residential use per capita of the population served in Canada is 215 litres per day. This equates to 47 Imperial gallons (56 USG) per person per day. Many softener manufacturers use 60 US gallons/person/day as the target amount for the purposes of calculating demand, so we will base our example on that figure.
Demand Example 1
We’ll assume a household with 5 people uses a water supply with 28 gpg of calcium carbonate equivalent. The calculation for softener demand would be:
[latex]28 \text{ gpg} \times 5 \text{ people} \times 60 \frac{\text{USG}}{\text{person}} \times 3 \text{ days} = 25\,200 \text{ grains of hardness}[/latex]
Demand Example 2
Supposing that the above water analysis showed that it also contained 0.5 mg/l iron and 0.25 mg/l manganese, the revised calculation for total hardness would be:
[latex]28 \text{ gpg}[/latex] + [latex]\left(\large 0.5 \frac{\text{ mg}}{l} \times 4 \frac{ \frac{\text{ gpg}}{\text{mg}}}{l} \text{ iron}\right)[/latex] + [latex]\left(\large 0.25 \frac{\text{ mg}}{l} \times 8 \frac{\frac{\text{ gpg}}{\text{mg}}}{l}\text{ manganese}\right)[/latex] = [latex]28 + 2 + 2 = 32 \text{ gpg total hardness}[/latex]
The revised demand calculation for the softener would be:
[latex]32 \text{ gpg} \times 5 \text{ people} \times 60 \frac{\text{UDG}}{\text{person}} \times 3 \text{ days} = 28\,800 \text{ grains of hardness}[/latex]
Manufacturers’ literature is then consulted for the selection of appropriately sized equipment.
Softener Sizing
Softener sizes will be expressed as grains of capacity they can hold at various salt settings, which can be manipulated with meter-initiated (digital) controls. For example, one manufacturer states that one of its models containing 1 cubic foot of resin, at the factory setting of 6 lbs salt per ft³ of resin, will hold 20000 grains of hardness. The same softener, when set at 10 lbs salt/ft³, will hold 27500 grains. Larger softener tanks will contain more resin, with the ability to exchange more hardness grains between regenerations. Varying tank sizes, variable salt settings and meter-initiated control valves allow fine tuning of efficiencies for salt and water usage and regeneration intervals.
Iron Filter Sizing
Calculation procedures for sizing iron filters will be like those in the examples above for softeners, with differing conversions particular to iron, manganese and hydrogen sulphide. Also, unlike the softeners, the regeneration frequency is not standard and will depend on the water conditions and equipment chosen. Both manganese greensand and chemical-free iron filters should be sized according to specific manufacturers’ literature.
 Now complete Self-Test 5 and check your answers.
Now complete Self-Test 5 and check your answers.
Self-Test 5
Self-Test 5
Media Attributions
- Figure 18. "Equipment suppliers' water sample container and form" from Pentair is used for educational purposes under the basis of fair dealing.
- Figure 19. "TDS meter" from AZGoodsDeals on Ebay is used for educational purposes under the basis of fair dealing.
- Figure 20. "Mixing container, water measuring tube and reagent causing colour change" are screenshots from Lancaster Water Group is used for educational purposes under the basis of fair dealing.
- Figure 21. "Iron test colour comparison equipment" from Hanna Instruments is used and adapted for educational purposes under the basis of fair dealing.
- Figure 22. "Colour comparator" from Lovibond™ is used and adapted for educational purposes under the basis of fair dealing.

