Competency C2: Install Residential Water Treatment Systems
Learning Task 1
Describe Water Treatment Equipment
The Water Cycle
All life on the planet depends on water for its existence, and the availability of potable (safe for human consumption) water for mankind to survive is dependent on the earth’s “water cycle”, also known as the “hydrologic” or “hydrological” cycle (see Figure 1).
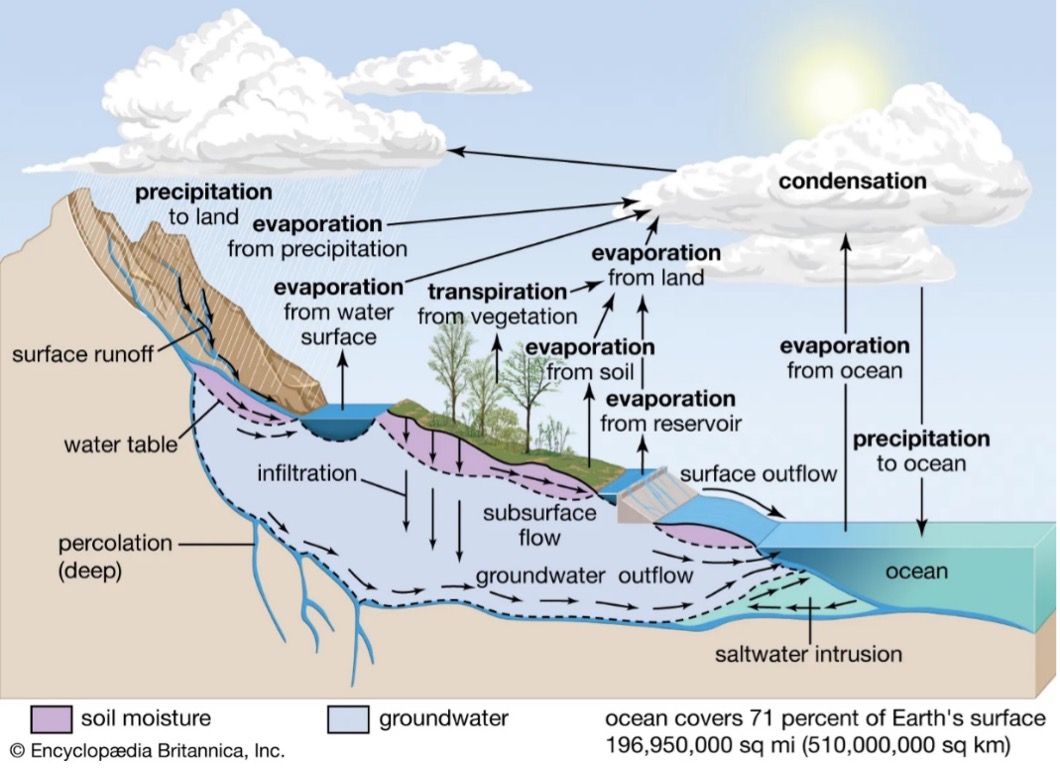
One way to describe the hydrological cycle of the earth is “the sum of all processes in which water moves from the land and ocean surface to the atmosphere and back again. The short form explanations of these processes are:
- precipitation – water is released from clouds as rain, snow or sleet
- condensation – water in the air condenses into small airborne particles to form fog, dew or clouds
- infiltration – water enters the ground by contact with rain or snow
- percolation – water from infiltration moves vertically through the ground by gravity and capillary action until it reaches an aquifer
- runoff – water that can’t be absorbed into the ground moves horizontally across it where it collects in bodies such as rivers, lakes or oceans
- evapotranspiration – also known as transpiration, this is water that is released into the atmosphere through plants as they “breathe”
- sublimation – the process whereby glacial ice and snow release water vapour without first melting into a liquid
- evaporation – liquid water when heated turns into a vapour, leaving behind any impurities such as minerals and chemicals
Water is continuously moving between the atmosphere and the earth through the processes listed above, and the only source of pure water within the water cycle is the earth’s atmosphere, where it is either invisible in low concentrations or can be seen in high concentrations as clouds. Once released from clouds as rain, snow, or a mixture of the two (sleet), pure water falls to the earth’s surface. On the way, it picks up any impurities that may be present in the air, such as dust, chemicals, gases, etc. This now impure water finds its way to various sources of water supplies such as lakes, rivers, and oceans, known as “surface water”. As well, it soaks into the ground to become part of an underground stream or lake called an “aquifer”. This water supply is known as “ground water”.
Surface Water Supplies
Although fresh water from lakes and rivers is relatively “soft” (free of dissolved minerals), it is prone to contamination from other sources. Bacteria and parasites from human and animal wastes, as well as chemicals from manufacturing and silt and debris (“turbidity”) from surface runoff are all possible components of surface water supplies.
Ground Water Supplies
As surface water percolates through the ground, many of the impurities it has collected are filtered out by the soil. However, when it meets carbon dioxide within the soil, carbonic acid is formed. This dissolves some of the mineral content of the soil or rock it contacts, in particular calcium and magnesium, thus adding these minerals to the water, which makes it “hard”.
The Need for Water Treatment (Water Conditioning)
Regardless of the source of supply, water will need some form of treatment before it can be considered potable. This learning guide will describe the different impurities that can be present in residential water supplies and the methods and equipment used to treat or remove them.
Common Impurities in Water
A molecule of pure water is made up of two atoms of hydrogen and one atom of oxygen (H2O), making it a compound. Water is called a universal solvent because it can dissolve much more substances, called solutes, than any other liquid found in nature; however, it cannot dissolve every substance. For example, water cannot dissolve fats, waxes, and hydroxides due to the low solubility of oppositely charged particles. Water molecules consist of a polar arrangement of two hydrogen atoms and one oxygen atom. The hydrogen atoms have a positive charge while the oxygen atom carries a negative charge. These polar charges help the water molecule to bind to different types of molecules. This is called “hydrogen bonding”, where the positive hydrogen of one water molecule will link with the negative oxygen of the next molecule, whose hydrogen atoms will then be attracted to the next oxygen, and so on. The attraction between the electrical charges in the water (solvent) and the substance (solute) cause the solute’s particles to be pulled apart and eventually dissolve. The rule of thumb is that “like dissolves like” and so a polar solvent like water can only dissolve solutes that also possess polar atoms, which are also known as ionic compounds. Ionic compounds are substances formed through chemical bonds between ions with opposite charges.
A good example of a polar solute is table salt (NaCl or sodium chloride). The sodium ions in sodium chloride have a partial positive charge, while the chloride ions have a partial negative charge. The sodium are attracted to the partial negative oxygen atoms of the water molecule, while the chloride ions are attracted to the partial positive hydrogen atoms of the water molecule. Eventually, this causes the atoms within the NaCl molecule to “pull apart” and dissolve into the water.
Dissolved Solids
Dissolved solids, known as “total dissolved solids”, or “TDS”, is an expression that represents the total concentration of dissolved substances in water. Common inorganic salts that can be found in water include calcium, magnesium, potassium and sodium, which are all “cations” (positively charged ions) and carbonates, nitrates, bicarbonates, chlorides and sulfates, which are all “anions” (negatively charged ions). Although the effects of TDS in water can be seen, such as staining of fixtures, the water may appear clear, and the presence and concentration of TDS can only be determined by analysis. Alone, a high concentration of dissolved solids is usually not a health hazard. However, high concentrations of solids such as calcium will influence piping and pressure vessels such as boilers and water heaters. A buildup of hardness minerals, called scale, on the inside walls of pipe can reduce water , and scale can cause a loss of efficient heat transfer in water heating appliances.
Suspended Solids
Suspended solids are substances like silt and clay, which aren’t ionic compounds and therefore don’t dissolve in water, so they stay in suspension and make the water murky and unappealing. This is called “turbidity” and is a characteristic of water that is visually identifiable. Over time, turbid water will cause a buildup of silt in storage containers in the water system, such as hot water tanks, boilers, and toilet tanks.
Gases
Gases such as hydrogen sulphide (H2S) and methane (CH4) can be dissolved and be present in ground water supplies. Although methane is tasteless, colourless and odourless, the same cannot be said of hydrogen sulphide. Even in very small concentrations, H2S has a rotten egg odour that makes tap water quite unappealing, and also quite corrosive. CH4 in water is much less noticeable but, in high enough concentrations, it can be an issue if allowed to come out of solution and collect.
Bacteria and Pathogens
Due to the filtering effect of the soil as water moves through it, deep wells are less prone to contamination from pathogens (disease-causing bacteria, viruses and protozoa) than are shallow wells. Animal or human waste (feces), runoff from industrial or residential developments, and runoff from landfills, septic fields and sewer pipes can carry pathogens into shallow ground water supplies. When these are found to be present in a water supply, it is of great concern and the situation must be rectified. Examples of waterborne infectious pathogens include Escherichia coli (E. coli), Campylobacter, Salmonella, Giardia and Cryptosporidium.
Acidity/Alkalinity
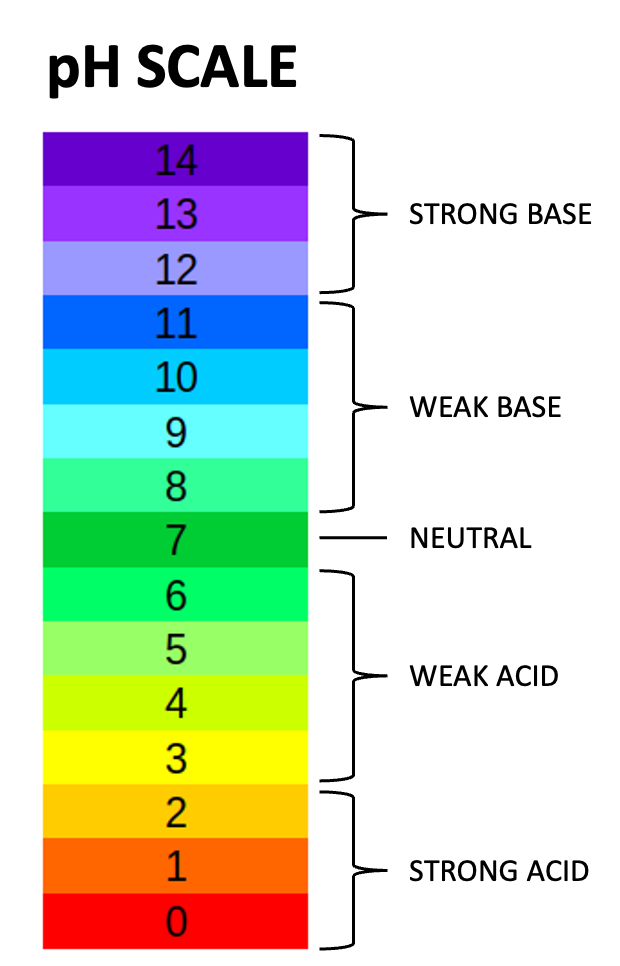
Both surface and ground water supplies can be found with levels of acidity or alkalinity. The term “pH” stands for “potential of hydrogen” which is a measure of the number or concentration of hydrogen ions in a substance. The pH scale is numbered from 0 to 14, with a pH of 7 being neutral. Pure water has a pH of 7; an acid will have a pH lower than 7 and an alkali or base will have a pH higher than 7. The further a pH number is from 7, the stronger an acid or alkali it is. Acidic water, which is the more common pH condition found in water supplies, can be treated by the introduction of an alkali, such as limestone, to the water. This brings the water’s pH level back to as near 7 as possible. A pH reading in excess of 8.5 could indicate contaminated water and generally requires bacteriological and chemical analysis.
Iron
Iron content in a water supply is generally found in deep wells, where it occurs as either ferrous or ferric iron. Ferric iron will oxidize in water, turning it a light shade of red, and is called “red water iron”. Water containing ferrous iron is clear and colorless because the iron is completely dissolved and is known as “clear water iron”. Although an essential element for good health, iron in water causes taste issues as well as staining of fixtures. When iron exists along with certain kinds of bacteria, a smelly biofilm can form. To survive, the bacteria use the iron, leaving behind a reddish brown or black slime that can clog plumbing and cause an offensive odor. This slime or sludge is noticeable in the toilet tank when the lid is removed.
Manganese
Considered a “cousin” to iron, manganese is found naturally in groundwater, but levels can be increased by human activities like steel production and mining. Manganese can turn the water a brown or rust color, cause black or brownish-black staining of faucets, sinks, or laundry, and make the water have an off-taste or odor. A manganese greensand filter is commonly used to counteract manganese in water.
Tannins
Tannins, also known as humic or fulvic acid, are created as water passes through rotting organic matter or peaty soil in the water table. They're also caused by low hanging branches, dead leaves, and trees that are exposed to a shallow well or surface water source. Because they naturally occur in low concentrations in drinking water, they are normally an aesthetic rather than a health concern, although their presence can alter the taste and appearance of potable water. While low levels of tannins can be removed through carbon filtration or cation exchange water softeners, high levels are handled through the use of anion exchange or whole-house reverse osmosis units.
Other Contaminants in Water Supplies
- Nitrate - Nitrate is the most common chemical contaminant in the world's groundwater and aquifers. Present in chemical fertilizers, human sewage, and animal waste and fertilizers, nitrate can contaminate a private well through groundwater movement and surface water seepage and run-off. Once taken into the body, nitrates are converted into nitrites which interfere with bodily functions.
- Heavy metals – Heavy metals can leach into drinking water from household plumbing and service lines, mining operations, petroleum refineries, electronics manufacturers, municipal waste disposal, cement plants, and natural mineral deposits. Heavy metals include: arsenic, antimony, cadmium, chromium, copper, lead, selenium and many more. Heavy metals can contaminate private wells through groundwater movement and surface water seepage and run-off.
- Organic chemicals - Organic (carbon-based) chemicals are found in many household products and are used widely in agriculture and industry. They can be found in inks, dyes, pesticides, paints, pharmaceuticals, solvents, petroleum products, sealants, and disinfectants. Examples include gasoline, plastics, detergents, dyes, food additives, natural gas, and medicines. Organic chemicals can enter ground water and contaminate private wells through waste disposal, spills, and surface water seepage and run-off.
- Fluoride - Fluoride can be present in many aquifers and can be found in private wells. The occurrence of fluoride in groundwater is due to weathering and leaching of fluoride-bearing minerals from rocks and sediments. Fluoride when ingested in small quantities is beneficial in promoting dental health by reducing dental caries (tooth decay or cavities), whereas higher concentrations may cause fluorosis, a condition characterized by pain and tenderness of bones and joints.
- Arsenic - Arsenic (As) is a naturally occurring contaminant found in many ground waters. Arsenic in water has no color, taste, or odor, and can only be measured by an arsenic test kit or lab test. Public water utilities must have their water tested for arsenic and if desired, the results can be obtained through the utility’s consumer confidence report. Private wells will need to have the water evaluated, and the local health authority can provide a list of test kits or certified labs.
There are two forms of arsenic: pentavalent arsenic (also called As (V), As (+5)) and trivalent arsenic (also called As (III), As (+3)). In well water, arsenic may be pentavalent, trivalent, or a combination of both. Although both forms of arsenic are potentially hazardous to health, trivalent arsenic is considered more harmful than pentavalent arsenic.
In summary, there is an expectation that surface and ground water sources will need some form of treatment due to water’s characteristics as a solvent. Obviously, preventive measures are paramount to maintaining a healthy water supply. Fortunately, when undesirable substances are detected in water supplies, there are means and methods available to treat water and make it potable.
 Now complete Self-Test 1 and check your answers.
Now complete Self-Test 1 and check your answers.
Self-Test 1
Self-Test 1
Terminology of Water Treatment
The following is a glossary of terms used in water treatment, some of which may be encountered within this learning guide.
- Activated carbon - Granulated active carbon, or “GAC”, used to remove tastes, odor, chlorine, chloramines, and some organics from water.
- Adsorption - The process by which molecules or colloids physically adhere (cling) to the surfaces of solids. Organic chemicals are adsorbed by carbon filters.
- Aeration - The process of adding air to a water supply for the purpose of oxidation. Aeration is frequently used to remove iron and hydrogen sulfide.
- Algae - Plant-like organisms which grow in water.
- Alkalinity - See Total Alkalinity.
- Anion – A negatively charged ion. Bicarbonate, chloride, and sulphate are the most abundant anions in water.
- Aquifer - Any geological formation containing water; one that supplies water for wells, springs, etc.
- Backwash - Reverse of a solution's flow through a system. Often used as a cleansing step in softeners and sand and dual media filters. Backwashing cleans and resettles the filter bed.
- Bacteria - Disease-potential organisms living in soil, water, or organic matter and being autotrophic (self-generative) or parasitic.
- Bromine – A chemical sanitizer that kills bacteria and algae.
- Buffer – A chemical that resists pH change, e.g., sodium bicarbonate.
- Calcium hardness - A measure of the calcium salts dissolved in water.
- Cation – A positively charged ion. Sodium, magnesium, potassium, and calcium are the most abundant cations in water.
- Chemical solution feeder - A pump used to meter chemicals such as chlorine or polyphosphate into a water supply.
- Chloramine - A combination of free chlorine and ammonia gas that retains its bactericidal qualities for a longer time than does free chlorine. It is less effective than chlorine as a disinfectant but is often used because it reduces the harmful by-product chemicals produced by free chlorine. It is becoming more common as the standard disinfectant used by municipal water supplies. In general, it is more difficult to remove from water than free chlorine.
- Chlorine – A chemical sanitizer that kills bacteria and algae. A very toxic biocide. A halogen element isolated as a heavy irritating greenish-yellow gas of pungent odor used especially as a bleach, oxidizing agent and a disinfectant in water purification.
- Chlorine demand – The amount of chlorine required to react on various water impurities before a residual is obtained.
- Chlorine, Free (Residual) - Chlorine available to kill bacteria or algae. Chlorine that has not combined with other substances in water.
- Contaminant - any physical, chemical, biological, or radiological substance or matter in water.
- Dl (deionization) – The use of ion exchange resin to remove salts from water.
- Demineralization - The process of removing minerals from water, e.g. deionization, reverse osmosis and distillation.
- Disinfection - Destruction of bacteria, viruses and cysts in a water supply or distribution system.
- Dissolved solids - Any minerals, salts, metals, cations or anions dissolved in water.
- Distillation - Steam from boiling water is condensed on a cool surface, collected, and stored. Most contaminants do not vaporize and therefore do not pass to the condensate. Removes nearly 100 percent of salts and organics.
- Ferric iron - Iron that is oxidized in water and is visible. Also called red water iron.
- Ferrous iron - Iron that is dissolved in water. Also called clear water iron.
- Flocculation - A water treatment process where solids form larger clusters, or flocs, to be removed from water. This process can happen spontaneously, or with the help of chemical agents.
- Flocculent chemical – A chemical which, when added to water, causes particles to coagulate into larger groupings (flocs), which can then settle from the water.
- GPD - Gallons per day.
- GPG - Grains per gallon. A grain is [latex]\frac{1}{7000}[/latex] of a pound. The grain per gallon (gpg) count is a unit of water hardness defined as 1 grain (64.8 milligrams) of calcium carbonate dissolved in 1 US gallon of water (3.78 L) which translates into 1 part in about 58000 parts of water, or 17.1 parts per million (ppm). Because an Imperial gallon is larger than a US gallon, one grain of hardness in 1 Imp. Gal. will have a lower concentration, which would be 14.3 ppm. This is the most common measurement for hardness.
- Ground water or groundwater - Water confined in semipermeable rock layers; in other words, well water.
- Hardness - The concentration of calcium and magnesium salts in water. Water hardness is responsible for most scale formation in pipes and water heaters and forms an insoluble “curd” when it reacts with soaps. Hardness is usually expressed in grains per US gallon (gpg), parts per million (ppm) or milligrams per liter (mg/l), all as calcium carbonate equivalent.
- Heavy metals - Metals having a high density or specific gravity. A generic term used to classify contaminants such as arsenic, cadmium, lead, and mercury.
- Hydrogen sulfide - A toxic gas (H2S) that is detectable by a strong "rotten egg" odor.
- Hydrologic cycle (hydrological cycle, water cycle) - The term used to describe how water travels through the environment by processes such as evaporation, condensation, and precipitation.
- Ion exchange - A chemical reaction in which ions are exchanged in solution. Water softening and deionization are common applications of ion exchange.
- Magnesium hardness - A measure of the magnesium salts dissolved in water.
- Membrane – A polymer film utilized as the semipermeable separation mechanism in reverse osmosis.
- Mg/l - Milligrams per liter. For our purpose, same as ppm. Normally used for a more accurate measurement or where small quantities of certain elements cause big problems in relation to iron, manganese, sulfur, nitrates and silica. There are 1000 mg in 1 gram, and 1000 grams in 1 litre, so 1000 mg × 1000 g = 1000000 mg in 1 litre.
- Micron - A unit of length, which is [latex]\frac{1}{1\;000\;000}[/latex] of a meter. It is the most common measurement of the size of particulate that a filter can trap. Filter sizes are designated in microns.
- Muriatic Acid - An acid used to reduce pH and alkalinity. Also used to remove stain and scale.
- Osmosis - The spontaneous flow of water from a less concentrated solution to a more concentrated solution through a semipermeable membrane occurring until energy equilibrium is achieved.
- Ozone - A powerful oxidizing agent with three atoms (O3) which, when dissolved in water, produces a broad-spectrum biocide that destroys all bacteria, viruses, and cysts. Normally used commercially for large-scale disinfection of water.
- Particulate - Minute, separate particles.
- Permeable - Allowing some material to pass through.
- Permeate - The portion of the feed stream that passes through the membrane. Also called "product water" of a reverse osmosis unit, meaning the finished water that you drink.
- pH - A measure of the acidity or alkalinity of water. The pH scale runs from 0 to 14 with 7 being the mid-point or neutral. A pH of < 7 is on the acid side of the scale with 0 as the point of greatest acid activity. A pH of ˃7 is on the basic (alkaline) side of the scale with 14 as the point of greatest basic activity. The pH value is an exponential function, so pH 12 is 10 times more alkaline than pH 11 and 100 times more alkaline than pH 10. Similarly, a pH 4 is 100 times more acidic than pH 6.
- PPM - Parts per million. One part dissolved material in one million parts of water. Used as a measurement for iron, manganese, TDS, hydrogen sulfide, chlorides, sulfates, and tannins.
- Regeneration - Refers to the process by which an ion exchanger (i.e., a water softener) or media filter renews its ability to do its job.
- Rejection - Material not being allowed to pass through a membrane. A reverse osmosis unit "rejects" contaminants and does not allow them to enter the permeate, or product water.
- Residual – Usually applying to chlorine in water, it is the amount of chlorine left after initial contact time that will provide disinfection. It is measured as mg/litre.
- Resin - Specially manufactured polymer beads used in the ion exchange process to remove dissolved salts from water.
- Reverse osmosis - The separation of one component of a solution from another component by means of pressure exerted on a semipermeable membrane. Reverse osmosis is a popular and effective drinking water treatment that reduces "dissolved solids" that are often not filterable by other means.
- Scale - Crust of calcium carbonate, which generally refers to calcium buildup in pipes or the interior of appliances like hot water heaters.
- Semipermeable - Able to allow certain size material to pass through while rejecting other size material. A reverse osmosis unit uses a semipermeable membrane.
- Soda ash – A chemical (sodium carbonate) used to raise pH and total alkalinity.
- Sodium bisulfate – Also called dry acid, it is a chemical used to lower pH and total alkalinity.
- Soft water - Water containing less than 17 PPM calcium or magnesium.
- Solute - Dissolved particles in a solvent.
- Superchlorination - Application of large dosages of chlorine to destroy build-up of undesirable compounds in water.
- Suspended solids - Small solid particles which remain in suspension in water, making it turbid (see Turbidity).
- Titration - A method of testing by adding a reagent of known strength to a water sample until a specific color change indicates the completion of the reaction.
- Total alkalinity - A measure of the acid neutralizing capacity of water which indicates its buffering ability (a measure of its resistance to a change in pH). Generally, the higher the total alkalinity, the greater the resistance to pH change.
- Total dissolved solids - The weight of solids, per unit volume of water, which are in true solution. It can be determined by evaporating a measured volume of filtered water and determining the residue’s weight. A common alternative method to determine TDS is to measure the water’s ability to conduct an electrical current through it.
- Turbidity - Muddy, clouded, stirred-up sediment, silt, clay, etc.
- Ultraviolet disinfection - The use of ultraviolet light to interrupt the DNA of, and sterilize bacteria, protozoa, and cysts.
Types of Water Treatment
Water quality standards in Canada are prescribed by Health Canada’s “Guidelines for Drinking Water Quality”. These guidelines are prioritized and developed in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water. The most significant risks to peoples’ health from drinking water come from microscopic organisms such as disease-causing bacteria, protozoa, and viruses. The guidelines that relate to these microorganisms are stringent because the associated short- and long-term health effects can be quite severe. Chemical and radiological substances may also be found in some drinking water supplies, but these are generally only a concern if they are present above guideline levels, with exposure to them over a period of years. However, new science is showing that exposure to some chemical contaminants above guideline levels may be a concern in the short-term as well. Aesthetic quality guidelines address parameters which may affect consumer acceptance of drinking water, such as taste, odour, and colour, and the choice to rectify aesthetic concerns is mainly left to the consumer.
The need for water treatment is established through the testing of water samples. The various tests and their procedures will be covered later in this learning guide.
Once a sample has been analyzed and a report of the test findings is generated, appropriate treatment methods and equipment can be prescribed. The following is a list of water treatment categories which will be covered in this text, although not necessarily in this order:
- Basic filtration
- Neutralization
- Ion exchange
- Oxidation
- Chlorination
- Distillation
- Ultraviolet sterilization
- Reverse osmosis
Filtration
Water filters come in a variety of forms. They range from small point-of-use (single fixture) cartridge-type to larger point-of-entry (whole-house) fibreglass tanks containing various types of filter media. Basic filtration involves the removal of sediment, silt or particulate that is large enough to be trapped by fine filtering media such as sand or fabric filters.
Cartridge-type filters
These are an inline variety and can be single units or two or more filters in series. Plastic housings containing a filter cartridge are intended to be installed on cold water lines only, whereas a hot water line would require a stainless steel housing. The maximum pressure that most manufacturers state they should be exposed to is usually 100 psi (700 kPa). Depending on the manufacturer, the filter housing (sump) is sometimes made of clear plastic so that the cartridge can be inspected visually for buildup. They can be supported by the pipe they are fitted to or mounted to a wall with a mounting bracket and hardware. A sump wrench is normally supplied with it for easy sump removal. Optional built-in valves on the inlet and outlet allow for isolation to enable quick cartridge changes, and a pressure-release button on the body relieves pressure from the housing prior to changing the cartridge. Filter assemblies come in various sizes with a range of maximum flow rates. The filter cartridges are designated by the size of particulate, in microns, that they can trap. Some types of cartridges, such as pleated polyester varieties, can be cleaned and re-used, however most cartridges are single-use and are discarded once full of sediment (loaded). Cartridge-type filters are normally considered point-of-use equipment and their intent is limited to treating screening dirt and sediment from the water, although activated carbon filter cartridges are also available for these units, to help in the reduction of bad tastes and odours and chlorine tastes and odours.


Whole-house filters
When whole-house treatment is desired, large fibreglass tanks containing filter media can be used. These allow higher flows rates through them than the cartridge-type, and the impurities they trap can be flushed to drain many times before the media needs to be replaced. Water conditions that can be treated by whole-house filter media include turbidity, iron, pH, manganese, hydrogen sulphide, chloramines, and taste/odour.

The media inside the fibreglass tank is particular to the condition being treated, for example, a base substance such as limestone is used to neutralize acidic water. There are normally three layers of material within the tank, which are;
- the media, forming the top layer
- coarse gravel as the bottom layer, and
- finer granular material sandwiched between the coarse gravel and media layers
There is some unused space, called “freeboard” above the top layer. This allows room for the filter media to expand or “fluff up” during the backwash cycle.
A distributor pipe connected to the valve assembly extends through the layers of media and fine gravel into the bottom of the coarse gravel layer at the bottom of the filter. In the service position (normal operation) water is directed from the valve head down through the distributor tube and through a screened fitting into the coarse gravel bed. The two gravel beds screen and trap particles of dirt and unwanted matter. As well, they help prevent the loss of filter media during the backwash cycle and also prevent channeling of water through the filter media. The screened water is treated as it flows upward through the media and exits the filter through the connected house piping. When it is time to regenerate the filter, the valve operates to reverse the direction of flow through the media and gravel which flushes the trapped particulate to the drainage system and rinses the gravel and media. Once this cycle is completed, the filter is once again in its “service position” and ready to go to work.

The timer and valve assembly, mounted to the top of the filter tank, can be either analog (oldest technology) or digital (more up to date). In most cases, the assembly is powered by the house electrical system, and a battery backup in the timer retains the programs in the event of a power outage. The valve head contains servo motors and valves that direct water through the filter and into the house piping when in its service position or through other positions such as “backwash” and “rinse” to periodically regenerate the filter.

Media for whole-house filters
Activated carbon
Activated carbon, or activated charcoal, is a porous substance produced from any base material that is carbonaceous (has a high percentage of carbon content), such as wood, nut pits or shells, animal bone, hydrocarbon sludge, peat, lignite, bituminous coal and anthracite coal. Carbonaceous material is first placed in an inert (without oxygen) tank and subjected to extremely high temperatures of between 800 - 900°C. This carbonized material, or char, must now be activated to fully develop the pore structure. This is done through oxidizing the char at temperatures again between 800-900 ºC but now in the presence of air, carbon dioxide, or steam. Once this process is complete, the activated carbon has a high surface area–to–volume mass ratio that allows it to adsorb organic compounds that produce taste, odour, colour, and toxicity issues, and can also reduce free chlorine present in the water supply.

Calcium carbonate
Calcium carbonate (CaCO3), also known as limestone, is a crushed and screened white marble material that is used in filters called neutralizers to adjust the pH of water that is in the range of pH 5.0 to 6.0. Acidic waters will slowly dissolve the limestone media, thereby raising its pH level to close to pH 7. The addition of calcium to the treated water may increase the hardness levels in the product water, and so may require the addition of a water softener for this reason. The calcium carbonate media will require periodic replacement depending on the incoming water’s pH and usage.
Magnesium oxide
Magnesium oxide (MgO) can also be used in neutralizers where more correction to acid water in the range of pH 4.0 to 6.0 is needed. However, in low flow conditions, magnesium oxide may cause overcorrection issues and turn the product water alkaline. For this reason, acid filters sometime use both calcium carbonate and magnesium oxide as the filter media. Magnesium oxide, like calcium carbonate, will need to be periodically replaced due to being dissolved through the neutralizing process.

Manganese greensand
Manganese greensand is used to remove soluble iron and manganese, as well as hydrogen sulphide, from water supplies. It is a purple-black filter media processed from glauconitic greensand. A solution of potassium permanganate (KMnO4) and water is periodically passed over the manganese greensand to regenerate it, in a process like that which is used in a water softener.

Sand
Sand is by far the most widely used filter media for water treatment, primarily to screen solid particles (sediment) from water. The sand must be of a type that is hard and sharp, normally produced through a crushing process, in comparison to river sand which normally has a rounded profile due to erosion. A sand filter can be found as a stand-alone component of a well water system that has much sediment and particulate to be removed, or as a component of a multi-media filter along with other substances such as anthracite and garnet to provide a broad-spectrum filter media.

 Now complete Self-Test 2 and check your answers.
Now complete Self-Test 2 and check your answers.
Self-Test 2
Self-Test 2
Filtration Continued
Iron filters
When iron is present in water in concentrations above that which can be treated with a softener, a separate iron filter can be used. There are two varieties, which are:
Manganese greensand filter – this has all the same components as a water softener, with the differences being:
- the substitution of magnesium greensand in place of the resin
- a potassium permanganate solution as the regeneration medium in place of sodium chloride brine, and
- a solution tank built specifically for the dry potassium permanganate agent
The filter goes through the same regeneration processes as does a softener.

Chemical-free iron filter – the tank in this variety is also similar to a softener tank, but unlike the manganese greensand filter there is no secondary tank holding a regeneration solution. Air is introduced into the water supply through an air induction valve, also known as a hydrocharger or air injector, as it travels through the valve head assembly on the filter. This starts the oxidation process, with the balance of oxidation occurring within the special filter material within the tank. Once oxidized, the iron precipitates out of solution and can be trapped in the filter bed, where it can be removed through the regeneration process.

Reverse osmosis filtration
The process of reverse osmosis (RO) is one of the finest levels of water filtration available for residential use. Depending on the manufacturer and size of unit, an RO unit will have either 3 or 4 vessel housings containing cartridge-type filters. The extra housing contains a pre-filter intended to remove suspended particulate that may be present. The first carbon filter, usually found on the upstream side of the membrane, is intended to remove chlorine from the water. The second carbon filter, called a post-filter, is mounted downstream of the membrane and is there to remove any taste or odour from the product water prior to delivery to the storage tank. The RO housing consists of a semi-permeable membrane with a very large surface area due to it being rolled into a cylindrical shape. According to many manufacturers, incoming water pressure must be at least 40 psi (280 kPa), with the optimum pressure being 50 – 75 psi (350 – 525 kPa). If water supply pressure is too low, units with a self-contained booster pump are available. The pressure of the incoming water pushes only pure water molecules through the membrane and into the post-filter housing. Depending on their molecular or atomic weight, various mineral salts, heavy metals, and particulate matter, as well as some organic molecules are repelled by the membrane’s surface and are rejected to the drainage system. The filtered water, called product water, is directed to the storage tank. RO units should only be applied to bacteriologically safe water supplies, as the membrane will not be effective to remove most bacteria and viruses.
Residential RO units are typically small capacity, delivering on average either 25, 50 or 75 US gallons of product water per day, and their membrane cartridges are colour-coded to indicate their daily capacity. RO units are used as point-of-use items and are most often located at kitchen sinks. The vessel housings and product water tank are installed within the sink’s cabinet, with a faucet (spigot) installed through the countertop and adjacent to a sink bowl. Because the unit is considered a cross connection (interconnected between the water supply and drainage system), the faucet must contain an air gap. This controls the cross connection and, provided the installer follows the manufacturer’s installation instructions, prevents backflow of drainage into the water supply.

Chlorination
When unwanted bacteria are present, a disinfecting agent such as chlorine can be added to kill the bacteria, provided it is in sufficient concentration and there is adequate contact time. As well, chlorine is an effective oxidizing agent, and so it can also be used to remove hydrogen sulphide. Chlorine is usually added to the water supply using a chemical feed pump, also known as a metering pump. This is a very precise operation, requiring accurate monitoring of the amount of chlorine injected to maintain a level that will be effective in disinfecting the water, which is termed “residual chlorine” and is measured in mg/l. Too much chlorine residual will create taste and odour issues as well as being corrosive to piping; too little will be a waste of chlorine and will not provide adequate disinfection. As well, chlorinated water can produce trihalomethanes (THMs). THMs are produced when chlorine reacts with organic matter, and there is concern among experts in Canada that THMs may pose a risk in the development of cancer. The simplest way to reduce or eliminate THMs in chlorinated drinking water is to use a water pitcher with a carbon filter, install a tap-mounted carbon filter or RO unit, or to use bottled water.

Distillation
The process of distillation involves the evaporation and condensation of water. When water is heated and turns to vapour, any dissolved impurities it contained are left behind. Capturing and condensing the water vapour results in pure water, which may still possess some objectionable taste and odour. These issues can be removed by passing the product water through a carbon filter. Distillation effectively removes bacteria, cysts, some chemicals, colour, and dissolved solids.

Ultraviolet “sterilization”
The most common means of disinfecting residential water is through the use of ultraviolet (UV) treatment. A specific spectrum of ultraviolet light emitted from one or more mercury vapour lamps inside a quartz-glass sleeve damages the DNA part of microorganisms so that they cannot reproduce. The five major groups of harmful microorganisms are viruses, bacteria, fungi, algae, and protozoa. Cells that can’t reproduce are considered dead, since they are unable to multiply to numbers considered infectious within a host. Both chlorine and UV treatment produces numbers of micro-organisms that are very low (approximately 99.9999% efficient) but not necessarily zero, and so although both processes are historically called sterilization, only high heat can truly sterilize a water supply. UV units are only effective if the water entering them has been filtered to a very high degree, as bacteria and other micro-organisms are so small that UV light can be blocked by particles of dirt or silt between the bacteria and the lamp.

 Now complete Self-Test 3 and check your answers.
Now complete Self-Test 3 and check your answers.
Self-Test 3
Self-Test 3
Ion Exchange Softeners
When the analysis of a water quality sample has determined that there is too much hardness (readings and their meanings will be covered in a later section of this learning guide), a water softener is usually prescribed. Water softeners work on the principle of ion exchange. Calcium and magnesium particles, the culprits behind hardness, possess a positive electrical charge. Left alone, they collect and form a layer called scale on the inside walls of pipes and vessels such as hot water tanks and boilers. Water softeners contain a bed of a substance known as resin. Today’s resin bed is made of small beads of plastic, whereas in decades past the resin was a natural volcanic material called zeolite that mainly consists of aluminum, silicon, and oxygen. In either case, the resin possesses a negative electrical charge. The softening process involves exposing the resin bed to a strong sodium chloride (NaCl) solution called brine. The positively charged sodium ions are attracted and cling to the resin and the softener is ready to do its job. As hard water ions pass through the resin bed, their attraction to the resin is stronger than that of the soft sodium ions, so the sodium is displaced into the water stream and out to the faucets, while the calcium and magnesium ions are held by the resin. When there are no more soft sodium ions left on the resin, the softener is exhausted and needs to be regenerated.
Water softeners have six basic control and valve settings, which are:
- service position
- backwash position
- brine position
- slow rinse position
- rapid rinse position, and
- brine tank refill
The service position
The “service” position refers to the state that the softener is in where hard water entering the softener is treated and soft water exits to the piping system supplying fixtures. In the service position, incoming hard water is directed through the valve head into the top of the tank, where it is pushed downward through the resin bed (downflow). This is where the exchange between hard calcium and magnesium ions in the water and soft sodium ions on the resin takes place. Softened water enters the distributor pipe at the bottom of the tank and travels upward through the valve head and out to the fixtures.
The other softener positions are part of the regeneration process.
The regeneration process
This refers to the steps necessary to be taken when the softener is no longer able to exchange soft ions for hard ions; in other words, the resin bed is depleted. In the regeneration process, the softener goes through several processes, which are backwashing, brining, and rinsing. As well, the salt tank is refilled with water to create a brine solution for the next regeneration cycle.
The backwashing position
Backwashing is simply pushing water in the reverse direction of flow through the resin bed. Water is directed from the head to the bottom of the tank through a vertical tube called the distributor pipe. Water exits the pipe through a nozzle which creates a swirling action. The swirling upward flow through the resin bed loosens suspended solids and impurities while expanding (“fluffing”) the resin bed up to 50% of its initial volume, preparing it for the next stage. The water then exits the top of the tank, taking the contaminants to the drain. This cycle takes about 10 minutes, at a flow rate of 4 – 8 GPM. If the softener is fed from a relatively clean municipal water system, it can be programmed to backwash less frequently.
The brining position
In a separate tank, sodium chloride (NaCl or salt) is dissolved into water to make a brine solution. Although potassium chloride (KCl) may also be used, this text will focus on sodium chloride solutions. In the “brining” position, brine is drawn from the brine tank through a venturi in the valve head and passed through the resin bed. If the brine concentration is strong enough, the hardness ions that have collected on the resin will be replaced with sodium ions, and the hardness ions removed from the resin, along with some excess sodium and chloride ions as well as any trapped particulate, are sent to the drain.
The brine flow through the resin can be either upward or downward depending on the model of the water softener. In downflow brining, the brine flows through the softener resin from top to bottom, in the same direction that the service flow occurs, and is termed “co-current”. In upflow brining, the brine flows through the softener from the bottom to the top, in the opposite direction of service flow, called “counter-current”. In general, upflow brining softener systems are more brine-efficient than downflow systems. This is because, at the beginning of a regeneration cycle, the resin highest in hardness is at the top of the bed, and the resin with the least amount of hardness is at the bottom of the bed. In downflow (co-current) regeneration, hardness that is exchanged off the resin during brining is pushed down the bed, where it continues to exchange on and off the resin until it’s pushed from the bed. The freshest brine is being used on the most depleted portion of the bed. In upflow (counter-current) regeneration, the hardness that is exchanged off the resin during brining is pushed back up the bed, exiting the system at the top of the bed. The freshest brine is being used to regenerate the least depleted portion of the bed. This highly regenerated portion of resin acts as a polisher which decreases hardness “leakage” (hardness ions left on the resin after regeneration). This allows an upflow regenerated softener to use, by some estimates, up to 75% less salt and up to 65% less water. The brining cycle takes about 30 minutes, at a flow rate of .05 to 1 GPM.
The slow rinse position
Once the brine draw is complete, fresh water is directed into the resin bed to give the resin a rinse. This flow moves slowly, allowing the ion exchange process to be complete. It removes the brine and sends it towards the drain. This cycle takes about 20 minutes, at a flow rate of .5 to 1.0 GPM. Besides brining, this is the only other process where the direction of flow is reversed in an upflow regeneration system. Direction of flow for backwashing and fast rinse are the same in both regeneration processes.
The fast rinse position
Once the slow rinse cycle is complete, a fast rinse cycle commences, flowing quickly through the resin bed from top to bottom. This rinse removes any remaining brine and hardness compounds and compacts the resin bed, preparing it for its service cycle. The cycle takes about 20 to 50 minutes, at a flow rate of 1.5 to 2.0 GPM.
The refill position
In this last stage before being returned to the service position, water is directed into the brine tank, where it dissolves some of the salt to create brine in preparation for the next brine draw cycle.
Other factors of softener operation
Regeneration efficiency
There are two measures of regeneration efficiency - brine efficiency and water efficiency. Brine efficiency is a measure of how much salt a softener system uses to remove hardness from the water. The brine efficiency of a system is calculated as the grains of hardness removal capacity per pound of salt used to regenerate the system (grain/lb). Water efficiency is a measure of how much water the system uses to regenerate, and is usually calculated as gallons per regeneration, or gallons of regeneration water per 1000 grains hardness removed.
Softeners that are tested to the “NSF/ANSI Standard 44 for Residential Cation Exchange Water Softeners” can be efficiency rated if they use demand-initiated regeneration. These will be digital softeners that “learn” the building’s water use and self-adjust regeneration frequency and duration accordingly. Analog or time clock softeners don’t have this ability and therefore cannot be efficiency rated. Per the standard, efficiency rated softeners must have a rated salt efficiency of at least 3350 grains per pound of salt used for regeneration. Efficiency rated softeners must also meet a water efficiency of 5 US gallons of regeneration water (or less) per 1000 grains of hardness removed.
To make the sodium exchange back onto the resin, a strong brine solution is required. To optimize brine efficiency, the system is designed so that as little excess sodium chloride as possible is discharged to the drain while still providing a solution strong enough to enable the ion exchange. Optimizing brine efficiency lowers system operating costs and reduces the level of brine discharged into the environment.
Variable Brining
Most digitally metered, single tank softeners delay regeneration until the night after the meter reaches its set capacity. To ensure that soft water is available throughout the last day, a portion of the softener’s capacity, equal to a day’s usage, is included in the softener’s sizing calculation. This is known as the reserve.
Variable brining is a control feature available on some upflow softeners whereby the controller determines how much reserve capacity has been used when the regeneration time is reached. Based on that remaining capacity, the system adjusts the salt dose used for that regeneration. This salt dose adjustment avoids using salt for resin that is still regenerated (not yet depleted). Fill time is varied to allow the salt dose to be matched to the actual amount of resin that is exhausted.
Variable brining will not work with downflow brining. With downflow brining, the first resin that is exposed to the brine is the most depleted. As that resin is regenerated, the hardness that is exchanged off the resin is pushed down the bed ahead of the more sodium-rich brine and will exchange onto less depleted resin. This resin will now be more fully depleted thus need to be fully regenerated. This continues all the way down the resin bed, so that resin with capacity remaining at the bottom of the bed will actually be depleted by the hardness ions being removed from the bed. This means that even if there is still some capacity remaining when regeneration starts in a downflow system, that capacity will be used by the time the full-strength brine reaches that portion of the resin bed.
Variable Reserve
Variable reserve is another means to minimize capacity wasted by the reserve setting. With a variable reserve system, the controller determines what the appropriate reserve for a system should be based on recent water usage patterns. This system increases and decreases the reserve capacity as required, helping to avoid both wasting salt and running out of soft water by optimizing reserve capacity.
Twin Tank Systems
Most softeners take approximately 2 hours to complete the regeneration process and the preferred time to start is 2am as there is normally little water use around that time. However, to maintain a water supply to fixtures during the regeneration process, the softeners engage an internal bypass feature that allows hard water to supply fixtures until the softener is ready to perform its duties once again. If there is a likelihood that there will be water used during this time of day and hard water to the faucets may be an issue, two softeners installed in parallel can alternate regeneration cycles so that there is always a supply of soft water to fixtures, regardless of time of day.
A twin tank system provides continuous soft water and only regenerates when the softener capacity is fully used. In a twin tank system, one tank is online and producing soft water while the other one is fully regenerated and waiting offline for its turn. When online tank capacity is reached, the offline tank is automatically brought online, and the depleted tank is taken offline and regenerated. Because regeneration can occur as soon as the capacity of the system is met, no reserve is required. The downside to this arrangement is the cost of the extra equipment.
 Now complete Self-Test 4 and check your answers.
Now complete Self-Test 4 and check your answers.
Self-Test 4
Self-Test 4
Media Attributions
- Figure 1. "The water cycle" from Encyclopædia Britannica is used for educational purposes under the basis of fair dealing.
- Figure 2. "pH scale" by Tux-Man on Wikimedia Commons, adapted by BCcampus, is in the public domain.
- Figure 3. "NOVO® Plastic and stainless-steel filter housings" from NOVO® is used with permission.
- Figure 4. "NOVO® Pleated, string wound, and activated carbon cartridges" from NOVO® is used with permission.
- Figure 5. "NOVO® Fibreglass whole-house media filter" from NOVO® is used with permission.
- Figure 7."NOVO® 485 digital valve" from NOVO® is used with permission.
- Figure 14. "RO unit filters, tank, and membrane"
- RO unit filters (Top Left): from AquaTru is used for educational purposes under the basis of fair dealing.
- RO tank (Top Right): from AquaTru is used for educational purposes under the basis of fair dealing.
- RO membrane (Bottom): by BCcampus is licensed under a CC BY-NC-SA licence, based on Schematic diagram of reverse osmosis based on the principle of cross flow filtration.
- Figure 15. "Chlorine feed pumps"
- Stenner Feed Pumps (Left): from StennerPumps is used for educational purposes under the basis of fair dealing.
- LV64SA-VTS8-PES (Right): from Pulsafeeder is used for educational purposes under the basis of fair dealing.
- Figure 17. "Combination UV/filter treatment unit" from from NOVO® is used with permission.
Image descriptions
- Figure 6. "Cutaway of whole house filter tank" image description: A mineral tank with a timer and control valve on top. Within the mineral tank, a distribution tube runs inside from the top to the bottom with a distributor at the bottom. Within the mineral tank (from the bottom to the top), there is a layer of coarse gravel, a layer of fine gravel, a thick layer of media or filter bed, and a layer of free board. [Return to Figure 6]
- Figure 8. "Calcium carbonate" image description: A mineral tank with a timer and control valve on top. Within the mineral tank, a distribution tube runs inside from the top to the bottom with a distributor at the bottom. Within the mineral tank (from the bottom to the top), there is a layer of coarse gravel, a layer of fine gravel, a thick layer of activated carbon, and a layer of free board. [Return to Figure 8]
- Figure 9. "Acidic neutralizer (acid filter)" image description: A mineral tank with a timer and control valve on top. Within the mineral tank, a distribution tube runs inside from the top to the bottom with a distributor at the bottom. Within the mineral tank (from the bottom to the top), there is a layer of coarse gravel, a layer of fine gravel, a thick layer of calcium carbonate and magnesium oxide, and a layer of freeboard. [Return to Figure 9]
- Figure 10. "Manganese greensand filter" image description: A mineral tank with a timer and control valve on top. Within the mineral tank, a distribution tube runs inside from the top to the bottom with a distributor at the bottom. Within the mineral tank (from the bottom to the top), there is a layer of coarse gravel, a layer of fine gravel, and a thick layer of magnesium green sand. Flowing into the control valve is a Potassium Permanganate Solution. [Return to Figure 10]
- Figure 11. "Multi-media filter" image description: A mineral tank with a timer and control valve on top. Within the mineral tank, a distribution tube runs inside from the top to the bottom with a distributor at the bottom. Within the mineral tank (from the bottom to the top), there is a layer of course gravel, a layer of fine gravel, a layer of coarse garnet, a layer of fine garnet, a layer of fine sand, and a layer of Anthrafilt (Anthracite). [Return to Figure 11]
- Figure 12. "Manganese greensand filter" image description: A mineral tank with a timer and control valve on top. Within the mineral tank, a distribution tube runs inside from the top to the bottom with a distributor at the bottom. Within the mineral tank (from the bottom to the top), there is a layer of coarse gravel, a layer of fine gravel, and a thick layer of magnesium green sand. Flowing into the control valve is a Potassium Permanganate Solution. [Return to Figure 12]
- Figure 13. "Chemical-free iron filter" image description: A mineral tank with a control valve on top. Flowing into the control valve are copper pipes that have an air vent and an air injector. Within the mineral tank, a distribution tube runs inside from the top to the bottom with a distributor at the bottom. Within the mineral tank (from the bottom to the top), there is a layer of coarse gravel, a layer of fine gravel, a thick layer of chemical-free iron filter media, and a layer of freeboard. [Return to Figure 13]
- Figure 16. "Cutaway of residential water distiller" image description: A labeled diagram of a residential water distiller, showcasing its main components. From top to bottom, the diagram highlights the activated carbon pre-filter, which removes impurities before distillation, the condensing coil, where steam cools and transforms back into liquid, and the boiling chamber, where water is heated to produce steam. It also includes the condensing fan, which aids in cooling, the heating element, responsible for boiling the water, the activated carbon post-filter, which further purifies the distilled water, and finally, the reservoir, where the clean, distilled water is collected. [Return to Figure 16]

