Chapter 6. Gases
35 Molecular Effusion and Diffusion
Jessie A. Key
Learning Objectives
- Explore the nature of gas movement: molecular effusion and diffusion.
- Examine and apply Graham’s law of effusion.
Effusion
The movement of gas molecules can be divided into a few different types. Effusion is the movement of gas molecules from one container to another via a tiny hole. Typically the container to which the gas is moving is kept under lower pressure.
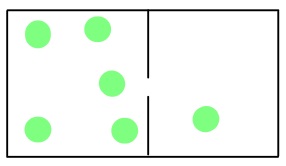
In 1846, the Scottish chemist Thomas Graham found that the rate of effusion of a gas (the amount of gas transferred between containers in a certain amount of time) is inversely proportional to the square root of its molar mass. This means that gases with a lighter molecular weight have higher effusion rates.

This finding is summarized in Graham’s law of effusion:
![]()
This finding can be rationalized by thinking through the process of effusion on the molecular level. For a gas molecule to successfully move from one container to another, it must hit and pass through the tiny hole present in the container. Gases with higher rms speed are more likely to hit and pass through the hole so effusion is dependent on rms speed:
![Rendered by QuickLaTeX.com \[\dfrac{\text{Rate of Effusion (Gas 1)}}{\text{Rate of Effusion (Gas 2)}}=\dfrac{\sqrt{\dfrac{3RT}{M_1}}}{\sqrt{\dfrac{3RT}{M_2}}}=\sqrt{\dfrac{M_2}{M_1}}\]](https://opentextbc.ca/introductorychemistryclone/wp-content/ql-cache/quicklatex.com-4d9aa9dc556404b74967d963434db2bb_l3.png)
Example 6.20
Problem
An unknown halogen (diatomic) gas effuses at a rate that is approximately 1.89 times the rate of I2 gas at the same temperature. Determine the molar mass and identity of this unknown gas.
Solution
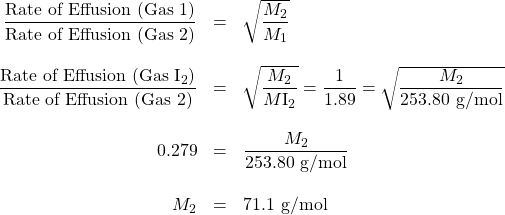
Therefore, the unknown gas is Cl2.
Diffusion
Another type of gas movement is called diffusion; it is the movement of gas molecules through one or more additional types of gas via random molecular motion. Similar to effusion, gases with lower molecular weights (which have a higher rms speed) diffuse faster than gases with higher molecular weights. However, in diffusion, movement is much more complicated as collisions occur between molecules that change the direction and speed of the molecules. As a result of these collisions, the path a molecule travels in diffusion is made up of numerous straight, short segments. The term mean free path is used to describe the average distance travelled by a molecule between collisions.
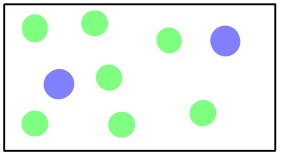
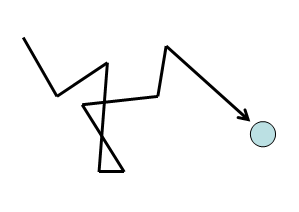
View this video on diffusion by Dr. Jessie A. Key for more information.
Key Takeaways
- Effusion is the movement of gas molecules from one container to another through a tiny hole.
- Rates of effusion can be compared at the same temperature using Graham’s law.
- Diffusion is the movement of gas molecules through one or more other types of gas via random molecular motion.
- Both the rates of effusion and diffusion are influenced by the molecular weight of the gas particle
Media Attributions
- “Molecular Effusion” by Jessie A. Key © CC BY (Attribution)
- “Thomas Graham” by Rudolph Hoffman © Public Domain
- “Molecular Diffusion” by Jessie A. Key © CC BY (Attribution)
- “Particle Mean Free Path” by DaisyDaisy © Public Domain
The movement of gas molecules from one container to another via a tiny hole.
A law that relates the rate of effusion of a gas to the inverse of the square root of its molar mass.
The movement of gas molecules through one or more additional types of gas via random molecular motion.
The average distance travelled by a molecule between collisions.

