Chapter 18. Psychological Disorders
Neurodegenerative Diseases
Leanne Stevens; Jennifer Stamp; Kevin LeBlanc (editors - original chapter); and Jessica Motherwell McFarlane (editor - adapted chapter)
Approximate reading time: 16 minutes
What is a Neurodegenerative Disease?
Neurodegenerative diseases vary in their causes, pathology, clinical symptoms, and treatment, which leads us to ask: What is considered a neurodegenerative disease? They are commonly characterised by gradual neuronal loss that can result in cognitive and motor impairments (Gao & Hong, 2008). Neurodegenerative diseases most often affect older individuals, and with an aging population these diseases will likely become more common (Brookmeyer et al., 2007). This section will describe the causes, clinical symptoms, and treatments of common neurodegenerative diseases including Alzheimer’s, Parkinson’s, Huntington’s, multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS). But what specific evidence do we have that these neurodegenerative diseases even exist in the first place?
Evidence for Neurodegenerative Diseases
Early Evidence
Before the age of neuroimaging, physicians and researchers studied neurodegenerative diseases with a more manual approach — post-mortem brain examinations. The basic idea was that if an individual showed pathological behaviour while they were living, observed abnormalities in their brain structure after they died could reveal physical evidence. Constantin Tretiakoff (Figure PD.24) was a young researcher working in France who made an important contribution to the understanding of Parkinson’s disease using this strategy. Tretiakoff compared the brains of individuals with Parkinson’s to healthy age-matched individuals, focusing on an area called the substantia nigra. Tretiakoff found that those with Parkinson’s showed a loss of neurons in this area, and suspected that these neurons contributed to the observable symptoms of the disease (Parent & Parent, 2010). This finding paved the way for further research in Parkinson’s by drawing a connection between observable symptoms and a specific region of the brain.

Modern Techniques
While post-mortem (after death) examinations are valuable in studying neurodegenerative diseases, neuroimaging techniques such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) are less invasive alternatives that can be performed on living people. Like post-mortem examinations, neuroimaging allows us to examine specific brain areas, but also allows for measurement of behaviour from living and conscious participants.
A major benefit of neuroimaging techniques is that they can be used to monitor brain physiology throughout the course of a neurodegenerative disease to better understand how it progresses. For example, researchers used MRI to scan the brains of patients with multiple sclerosis (MS) to examine the potential relationship between the amount of grey matter in the cerebellum and scores on cognitive and motor tests. They found that low levels of grey matter in specific areas of the cerebellum were linked to impairments in cognitive and motor test scores (Grothe et al., 2017). This study shows how neuroimaging can be used to establish a connection between observable symptoms and specific brain areas, making it a valuable tool for understanding neurodegenerative diseases.
Alzheimer’s Disease
Clinical Symptoms
Alzheimer’s disease is associated with many symptoms that can appear at different stages for different individuals, but ultimately all symptoms become more severe as the disease progresses (Sheppard & Coleman, 2020). Memory impairment is the primary cognitive symptom of Alzheimer’s, with some forms, like semantic memory, more severely affected than others, like procedural memory. Other symptoms include deficits in attention and language, loss of posture and facial expression control, and often a variety of sleep issues (Scarmeas et al., 2004; Sprecher et al., 2017). Sleep issues and memory deficits are some of the first signs of the disease, with symptoms becoming more prominent over time (Sprecher et al., 2017, Gold & Budson, 2008). Alzheimer’s disease is diagnosed most frequently after the age of 60, potentially affecting up to 50% of people over the age of 85 (Schachter & Davis, 2000).
The clinical symptoms of Alzheimer’s have strong financial implications, since many diagnosed with the disease require intensive assisted living at some stage (Brookmeyer et al., 2007). The Alzheimer Society of Canada projects national spending on Alzheimer’s-related costs to surpass $20 billion in the next ten years (Alzheimer Society of Canada, 2018). In addition to financial strain, Alzheimer’s disease can be stressful for caregivers. These people are often spouses or children who must watch their loved ones gradually lose cognitive function as the disease progresses (Schachter & Davis, 2000). Primary caregivers for individuals with Alzheimer’s are highly susceptible to depression, with women being disproportionately affected compared to men (Alqahtani et al., 2018; Chen et al., 2020).
Causes
A defining feature of Alzheimer’s disease is the presence of extracellular amyloid plaques found between neurons and intracellular tau tangles found within neurons (Bloom, 2014). Amyloid plaques are like sticky gunk that builds up between the brain cells, making it hard for messages to travel smoothly between different cells. Tau tangles, on the other hand, are like twisted-up wires inside the brain cell. They get all tangled and mixed up, making it difficult for the brain cells to communicate properly with each other. So, in Alzheimer’s, these plaques and tangles disrupt the normal functioning of the brain, causing memory loss and other problems. Plaques and tangles are also found in the brains of people without cognitive symptoms, but at much lower levels and in anatomically distinct areas of the brain (Price et al., 1997; Nelson et al., 2007). So how do plaques and tangles cause problems in some individuals and not others?
Specifically, when neurons express high levels of amyloid proteins, they clump together to form plaques between neurons in the medial temporal lobe (Sheppard & Coleman, 2020), while tau protein builds up to form tangles inside of the neurons. The build-up of these proteins (Figure PD.25) affects primarily cholinergic neurons (neurons that transmit acetylcholine), causing synaptic dysfunction and neuronal death (Ferreira-Vieira et al., 2016). The neurotransmitter acetylcholine is important for cognitive functions such as memory; damage to the neurons that produce it is linked to cognitive impairment in Alzheimer’s patients (Ferreira-Vieira et al., 2016).
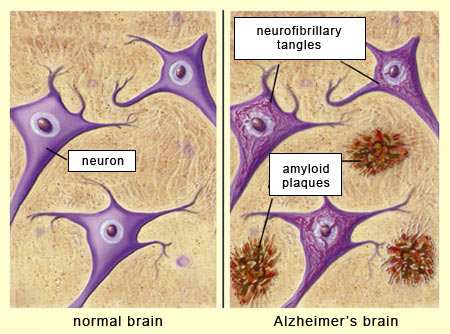
There are several genetic risk factors associated with developing Alzheimer’s disease, especially the early onset type that tends to run in families (Shastry & Giblin, 1999). Having one or more copies of the APOE-e4 allele (a different version of a gene that you can inherit from your parents) of the APOE gene makes a person more susceptible to developing the disease (National Institute on Aging, 2017). Some populations are more likely to possess copies of the APOE-e4 allele and show different vulnerabilities to developing symptoms. For example, people of African heritage who possess the APOE-e4 allele are less likely to be affected by it than those of European heritage (Serrano-Pozo et al., 2021). Although genes like APOE-e4 appear to be involved in symptoms of early onset Alzheimer’s disease, this accounts for less than 5% of cases (Shastry & Giblin, 1999). The majority of Alzheimer’s cases are later onset and not as tightly linked to family history, so the role of inherited genes in these cases is less clear.
Some of the strongest predictors of Alzheimer’s risk are related to environment and lifestyle, such as cognitive activity and verbal ability. One of the most surprising findings comes from the Nun Study on aging and Alzheimer’s disease (Snowdon, 2001). Participants in this longitudinal study were Catholic nuns from the School Sisters of Notre Dame who completed annual physical and cognitive tests to monitor possible symptoms of dementia. They also generously agreed to donate their brains when they died for detection of Alzheimer’s neuropathology, like plaques and tangles.
Linguistic analysis of their writing as young adults when they entered the sisterhood, showed that “idea density”, the average number of ideas expressed per ten words, predicted both cognitive impairment and neuropathology 50 years later (Riley et al., 2005)! Although this research is correlational and does not provide a direct causal link between cognitive activity as a protective factor, other research points to benefits of a busy brain. Another large-scale study showed that engaging in regular cognitive activity, like reading the newspaper, and doing crossword puzzles, was associated with a significantly reduced risk of Alzheimer’s-type memory symptoms five years later (Wilson et al., 2022).
Earlier Detection of Alzheimer’s Disease Using Biomarkers
Dr. Sultan Darvesh (Figure PD.26) and his research group are developing ways to detect Alzheimer’s disease in the early stages, by looking for biomarkers, molecules produced during pathological processes. Plaques and tangles are present in most people’s brains, but they only are linked to dysfunction in those who develop Alzheimer’s disease (Freer et al., 2016). Identifying differences in proteins associated with known neuropathology is a promising strategy (National Cancer Institute). For years researchers have tried to find a reliable biomarker of Alzheimer’s; recently, Darvesh and his team discovered that butylcholinesterase-associated plaques appear in the brains of individuals with Alzheimer’s disease, but not in the brains of patients diagnosed with other neurodegenerative diseases. Butylcholinesterase is an enzyme responsible for breaking down acetylcholine (which we know is negatively impacted in individuals with Alzheimer’s disease). This may be the first step towards earlier diagnosis and treatments for those living with Alzheimer’s.

In recent years, Darvesh’s group examined brains with and without plaques, and brains from individuals with and without Alzheimer’s. Researchers found butylcholinesterase plaques were present almost exclusively in the brains of individuals who had Alzheimer’s, while amyloid plaques were found in the brains of those with and without Alzheimer’s disease. Furthermore, butylcholinesterase plaques were not associated with any other neurodegenerative diseases, making them ideal biomarkers unique to Alzheimer’s (MacDonald et al., 2017).
Many neuroimaging techniques (e.g., PET scans) use radioactive molecules to identify biomarkers in the brain. These radioactive molecules need to be able to bond to the biomarker of interest so that researchers and clinicians can visualise where these biomarkers might be in the brain. Darvesh and his team are currently working to develop this type of molecule to allow for a high-resolution visualisation of plaques in the brains of Alzheimer’s patients. The biomarker may allow for earlier detection and potentially become a reliable tool for diagnosis.
More About Alzheimer’s Disease
Watch this interview with Dr. Sultan Darvesh, discussing his work on Alzheimer’s disease, to learn more.
Treatment
A cure for Alzheimer’s does not exist, meaning that those diagnosed will have the disease until death. However, there are drug-based and non-drug-based treatment options that can reduce some of the symptoms of the disease and aim to increase quality of life for Alzheimer’s patients; these are somewhat effective at reducing symptoms (e.g., memory loss, language difficulty).
Cholinesterase inhibitors (Study hint. chemicals that end in “ase” are enzymes which have the role of breaking down a neurotransmitter or other proteins) are a popular drug-based treatment that aims to prevent the breakdown of acetylcholine, a neurotransmitter crucial to many cognitive processes including memory. The inhibitors allow acetylcholine to remain in the synapse for longer, enhancing its effects (Larik et al., 2018). Another drug-based option is the use of NMDA antagonists which regulate the release of glutamate in the brain.
In Alzheimer’s patients, excessive amounts of glutamate can cause neuronal cell death which contributes to the symptoms of the disease (Liu et al., 2019). NMDA antagonists and cholinesterase inhibitors both work to reduce cognitive symptoms of Alzheimer’s disease but in different ways; as such, patients are often prescribed a combination of both of these types of drugs.
Besides prescribed drugs, Alzheimer’s disease can be managed through strong social support. Specifically, clinical psychologists recommend a psychosocial approach on its own, or in addition to drug-based treatment. Psychosocial interventions can include cognitive training, group exercise, and art or music therapy. Duan et al. (2018) conducted a meta-analysis on the effectiveness of several psychosocial interventions such as a walking program and group exercise. They looked at the effectiveness of these interventions on their own and when paired with cholinesterase inhibitors. A walking program, home-based exercise, and group exercise, among others, were found to improve the mental state of participants compared to those in the control condition. However, a combination of cholinesterase inhibitor and psychosocial intervention was found to be the most effective, more so than either treatment on their own (Duan et al., 2018).
Jingle Therapy for Alzheimer’s Disease
Jingle therapy is an innovative approach to reminiscence therapy for individuals living with Alzheimer’s disease. It involves the use of familiar commercial jingles, such as advertising slogans or product melodies, from different periods of a person’s life to evoke memories and stimulate cognitive function (Smith & Jones, 2020).
During jingle therapy sessions, patients listen to recordings of these jingles in a comfortable and supportive environment. The goal is to trigger specific memories and emotions associated with the jingles, promoting reminiscence and cognitive engagement. Research has shown that music can have a powerful effect on memory recall and emotional well-being, particularly in individuals with Alzheimer’s disease (Brown et al., 2018).
Therapists carefully select jingles based on the patient’s personal history and cultural background, aiming to create a meaningful and nostalgic experience. Patients are encouraged to share their thoughts and feelings evoked by the music, fostering social interaction and emotional connection.
While jingle therapy is still an emerging approach, preliminary studies have shown promising results in improving mood, stimulating cognitive function, and enhancing overall quality of life for individuals with Alzheimer’s disease (Johnson et al., 2019).
Image Attributions
Figure PD.24. In the Public Domain.
Figure PD.25. Figure 1 as found in Alzheimer’s Disease and the Aging Brain: A Review of Current Etiology and Treatments is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International.
Figure PD.26. Figure PY.26 by Dalhousie University as found in Introduction to Psychology & Neuroscience (2nd Edition) is licensed under a CC BY 4.0 License.
To calculate this time, we used a reading speed of 150 words per minute and then added extra time to account for images and videos. This is just to give you a rough idea of the length of the chapter section. How long it will take you to engage with this chapter will vary greatly depending on all sorts of things (the complexity of the content, your ability to focus, etc).

