21 Uniform Electric Fields
Resources
- Video to Watch: Mechanical Universe – Episode 30 Capacitance & Potential
- Video to Watch: Mechanical Universe – Episode 31 Voltage, Energy & Force
- Video to Watch: Mechanical Universe – Episode 32 Electric Battery
- Extra Help: A-Level Physics Tutor
Equations Introduced and Used in this Topic:
- [latex]\vec{E}=\dfrac{V}{\vec{d}}[/latex]
- [latex]F_{el}=q\vec{E}[/latex]
[latex]W=\Delta E=qV\text{ or }q\vec{E}\vec{D}[/latex]
Where…
- [latex]F_{el}[/latex] is the Electric Force, measured in newtons (N)
- [latex]V[/latex] is the voltage, measured in volts (V)
- [latex]\vec{E}[/latex] is the Electric Field, measured in newtons per coulomb (N/C) or volts per metre (V/m)
- [latex]q[/latex] is the Charge, measured in coulombs (C)
- [latex]∆E[/latex] is the Energy, measured in joules (J)
- [latex]d[/latex] is the Parallel Plate Separation, measured in metres (m)
- [latex]W[/latex] is the Change in Energy of any particle moving up or down the field lines
21.1 Energy Change of Charge moving through a Uniform Electric Field
Equations Introduced:
[latex]ΔE = qV[/latex]
Energy Unit: One electron volt (eV) is the energy gained by one electron or proton that is accelerated through a potential difference of one volt.
[latex]1\text{ eV}= 1.602 \times 10^{−19}\text{ J}[/latex]
Historical Development
The electric field created between two parallel charged plates is different from the electric field of a charged object. A proper discussion of uniform electric fields should cover the historical discovery of the Leyden Jar , leading to the development of capacitors and, in later works, parallel charged plates, which have been central to many developments in physics.
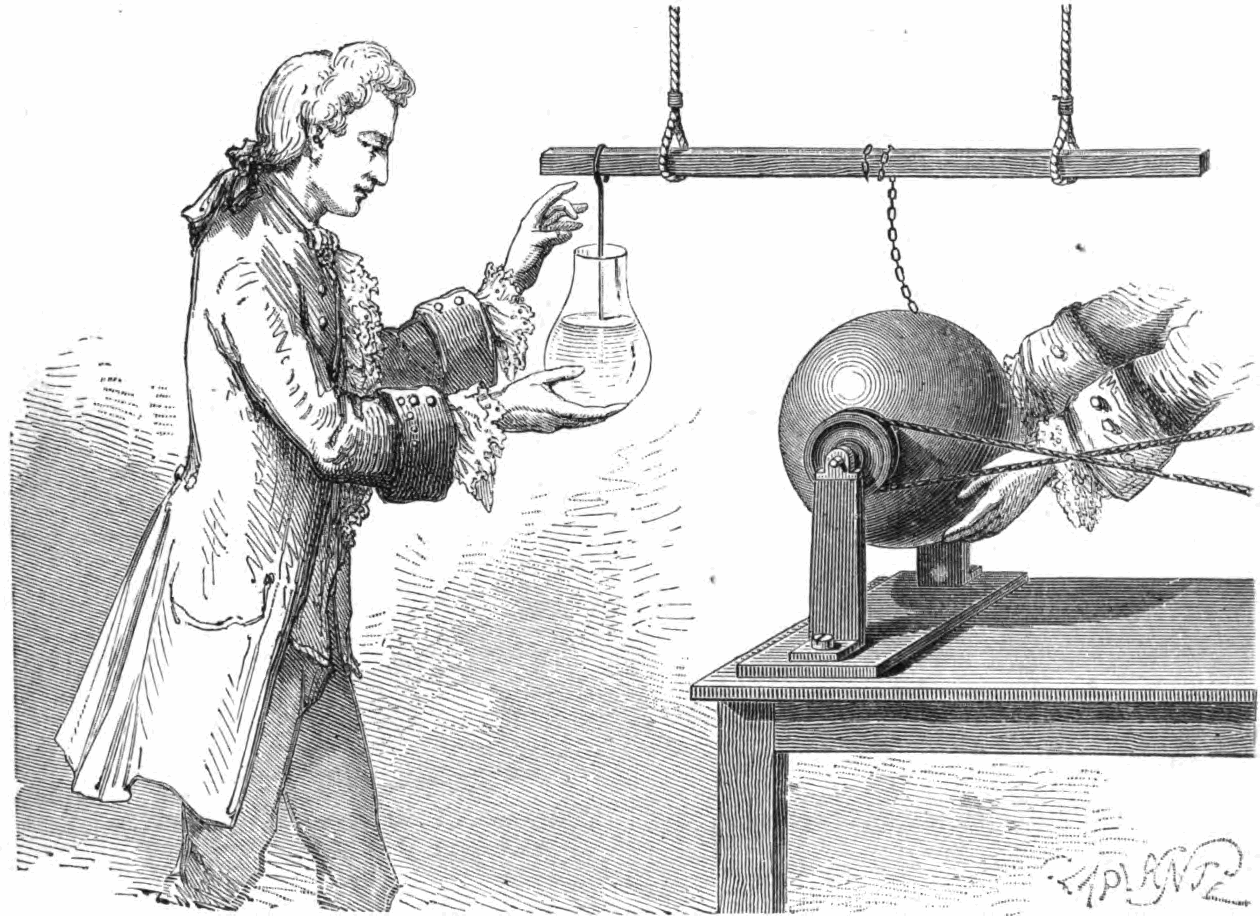
The ability to store electric charge was accidentally discovered in 1746, by Andreas Cunaeus, a lawyer visiting the laboratory of Pieter von Muschenbroek (1692-1761) in Leyden, Holland. This discovery arose from Cunaeus’s attempts to “condense” electricity into a glass filled with beer and who had failed to properly place himself on an insulated stool. The charging device was a rotating glass sphere that generated a static charge by the hands rubbing it, which was then transferred through a chain to a suspended metal bar, and then into a hanging wire into the hand-held glass of beer. This arrangement allowed the glass to build up a large charge in the beer, with an equal and opposite charge built in Cunaeus’s hand holding the glass. When Cunaeus began to pull the wire out of the beer he received a severe shock, far worse than the electrostatic machine could give. It took Cunaeus two full days to recover from this accident.
Musschenbroek was so distressed by the severity of the shock he later received from the device that he wrote, warning of the dangers of this device, “I would not take a second shock for the crown of France”. Reports of the experiment became widely circulated, and scientists began to investigate the charge storage ability of these “Leyden Jars”. Eventually the water that filled the jar was replaced by foil coatings on both the inside and outside of the jar.
Electric charge storage devices are suspected to have been created far earlier than the Leyden Jar in 1746. Nicholas Tesla speculated in a 1915 article that the Ark of the Covenant was in fact a capacitor, deadly to touch… “The records, though scanty, are of a nature to fill us with conviction that a few initiated, at least, had a deeper knowledge of amber phenomena. To mention one, Moses was undoubtedly a practical and skillful electrician far in advance of his time. The Bible describes precisely, and minutely, arrangements constituting a machine in which electricity was generated by friction of air against silk curtains, and stored in a box constructed like a condenser. It is very plausible to assume that the sons of Aaron were killed by a high-tension discharge, and that the vestal fires of the Romans were electrical.”
The Leyden Jar became one of great importance in the early development of the knowledge of static electricity since, before this, no device had been created that allowed for the storage of any significant amount of electricity. These early devices allowed for the storage of between 20 000 and 60 000 volts, which had varying amounts of energy, all depending on the amount of the charge stored. It was such a device that Benjamin Franklin used with his “famous kite in a thunderstorm” that was used to store the electric charge of part of a lightning bolt.
By the 1850s, the Leyden Jar was in quite common by researchers who were aware of the significant risks involved. Soon, the Leyden jar was used in spark-gap transmitters and electrotherapy, and was soon replaced by the more versatile capacitor.
The physics equation used for the simplest case of the constant electric field created in the storage of electric charge in a capacitor is as follows:
[latex]\vec{E}=\dfrac{V}{\vec{d}}[/latex]
This equation is valid for the central portion of very large parallel charged plates as the electric field is distorted near the ends or boundaries of the plates. As in electrostatic fields the electric field is measured in N/C, voltage (or Potential Difference between the parallel charged plates) is measured in volts and the distance separating the parallel charged plates is measure in metres. The negative sign in this equation is a relic of the definition of the movement of positive charge, since positive charges would be repelled by a positively charged plate. The following examples concern themselves with the energy contained in a constant electric field:
Example 21.1.1
If the potential difference between a thundercloud and the ground is 8.0 MV (megavolts) find the energy transferred to the ground if the first lightning stroke transfers 60 C of charge.
Solution
- [latex]∆E = qV[/latex]
- [latex]\Delta E[/latex] = (60 C)(8.0 × 106 V)
- [latex]\Delta E[/latex] = 4.8 × 108 J
Example 21.1.2
How much energy is transferred from one object to another, if a spark of 0.280 µC jumps from between them, where a 400 kV potential difference exists between the two objects?
Solution
- [latex]∆E = qV[/latex]
- [latex]\Delta E[/latex] = (0.280 × 10−6 C)(400 × 103 V)
- [latex]\Delta E[/latex] = 0.112 J
Example 21.1.3
A proton is accelerated through a potential difference of 28 000 V. What is its gain in kinetic energy?
Solution
- [latex]∆E = qV[/latex]
- [latex]\Delta E[/latex] = (1.602 × 10−19 C)(28000 V)
- [latex]\Delta E[/latex] = 4.5 × 10−15 J or 28000 eV
Example 21.1.4
What is the difference in kinetic energy of a proton and an electron accelerated through the same potential difference of 15 kV?
Solution
Both gain the same kinetic energy…
Data:
- [latex]\Delta E_k[/latex] = Find
- [latex]q[/latex] = 1.602 × 10−19 C
- [latex]V[/latex] = 15000 V
Solution:
- [latex]∆E_k = qV[/latex]
- [latex]\Delta E_k[/latex] = (1.602 × 10−19 C)(15000 V)
- [latex]\Delta E_k[/latex] = 2.4 × 10−15 J or 15000 eV
Therefore the difference between these two charged particles is zero!
Exercise 21.1
- If the potential difference between a thundercloud and the ground is 7.0 MV (megavolts), find the energy transferred to the ground if the first lightning strike transfers 50 C of charge.
- How much energy is transferred from one object to another, if a spark of 0.45 µC jumps from between them caused by the build-up of a 0.50 MV potential difference between the two objects?
- A proton is accelerated though a potential difference of 15000 V. What is its gain in kinetic energy?
- What is the difference in kinetic energy of a proton and an electron accelerated through the same potential difference of 5 kV?
21.2 Uniform Electric Fields (Parallel Charged Plates)
Equations Introduced or Used for this Section:
- [latex]{F}_{el}=q\vec{E}[/latex]
- [latex]W=\Delta E=qV\text{ or }q\vec{E}\vec{D}[/latex]
One of the interesting outcomes arising from the discovery of the constant electric field between two charged plates is the motion of charged particles moving between them. For these parallel charged plates the electric field is quantified as:
[latex]\vec{E}=\dfrac{V}{\vec{d}}[/latex]
This electric field is roughly constant between the two plates, which when combined with electric force can be used to cover both linear and projectile motion, impulse, momentum, kinetic and potential energy. The equations used are nearly identical to those used in previous topics, but one must be mindful that these only work in non-relativistic settings where v < 0.1 c, which at this speed generally amounts to a 1% error. Consider a negative charged particle between the parallel charged plates shown below:
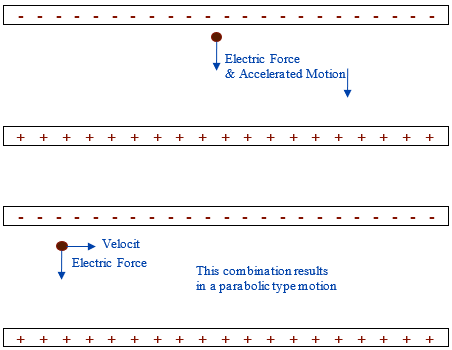
If the negative charge is stationary, then it will accelerate towards the positive plate using similar equations to what was used in vertical motion problems.
If the negative charge is moving at 90° to the direction of the electric field then, the motion of the negative charged object will be one that is parabolic using equations similar to what was used in the study of parabolic motion.
To analyze the motion of charged particles in a constant electric field, the electric force law is used in conjunction with the one or more of the accelerated motion or constant velocity equations. Expect to see combinations of the equations below used with this topic in future courses.
Four Kinematic Equations:
- [latex]\vec{d}=\dfrac{(\vec{v_i}+\vec{v_f})t}{2}[/latex]
- [latex]2\vec{a}\vec{d}=\vec{{v_f}^2}-\vec{{v_i}^2}[/latex]
- [latex]\vec{v_f}=\vec{v_i}+\vec{a}t[/latex]
- [latex]d=\vec{v_i}t+\dfrac{1}{2}\vec{a}t^2[/latex]
Electric Force:
[latex]{F}_{el}=q\vec{E}[/latex]
Constant Velocity:
[latex]\vec{v}=\dfrac{\vec{d}}{t}[/latex]
Example 21.2.1
If a potential difference of 50.0 V is applied across two parallel plates producing an electric field of 400 N/C (or V/m) what distance separates these two plates?
Solution
- [latex]\vec{E}=\dfrac{V}{\vec{d}}[/latex]
- 400 N/C = [latex]\dfrac{50.0\text{ V}}{\vec{d}}[/latex]
- [latex]\vec{d}[/latex] = 50.0 V ÷ 400 N/C
- [latex]\vec{d}[/latex] = 0.125 m
Example 21.2.2
An electric field of 450 N/C exists between two parallel charged plates separated by 20 cm. What is the potential difference that is causing this?
Solution
- [latex]\vec{E}=\dfrac{V}{\vec{d}}[/latex]
- 450N/C = [latex]\dfrac{V}{0.20\text{ m}}[/latex]
- [latex]V[/latex] = (450 N/C)(0.20 m)
- [latex]V[/latex] = 90 V
Example 21.2.3
What is the electric field that could be created by two parallel charged plates separated by 5 cm with a potential difference of 40 V between them?
Solution
- [latex]\vec{E}=\dfrac{V}{\vec{d}}[/latex]
- [latex]\vec{E}=\dfrac{40\text{V}}{0.05\text{ m}}[/latex]
- [latex]\vec{E}[/latex] = 800 N/C
Example 21.2.4
What is the energy gained by an electron that moves 5.0 cm through a 4000 N/C electric field?
Solution
Data:
- [latex]\vec{E}[/latex] = 4000 N/C
- [latex]\Delta E[/latex] = Find
- [latex]d[/latex] = 0.050 m
- [latex]q[/latex] = 1.602 × 10−19 C
Solution:
- [latex]ΔE = qV\text{ or }q\vec{E}\vec{D}[/latex]
- [latex]\Delta E[/latex] = (1.602 × 10−19 C)(4000 N/C)(0.050 m)
- [latex]\Delta E[/latex] = 3.36 × 10−18 J (≈ 3.4 × 10−18 J) or 21 eV
Example 21.2.5
What is the gain in the speed reached by a proton that is accelerated from rest through a potential difference of 15000 V?
Solution
First… The energy gained is:
- [latex]∆E = qV[/latex]
- [latex]\Delta E[/latex] = (1.602 × 10−19 C)(15000V)
- [latex]\Delta E[/latex] = 2.4 × 10−15 J
Since this is kinetic energy…
- [latex]E_k = \dfrac{1}{2}mv^2[/latex]
- [latex]2.4 \times 10^{−15}\text{ J}=\dfrac{1}{2}(1.67\times 10^{−27}\text{ kg})v^2[/latex]
- [latex]v^2=2.4\times 10^{−15}\text{ J}\div\dfrac{1}{2}(1.67 \times 10^{−27}\text{ kg})[/latex]
- [latex]v[/latex] = 1.7 × 106 m/s
Exercise 21.2
- If a potential difference of 20 V applied across two parallel plates produces an electric field of 500 N/C (or V/m) what distance separates these two plates?
- An electric field of 4200 N/C exists between two parallel charged plates separated by 15 cm. What is the potential difference that is causing this?
- What is the electric field that could be created by two parallel charged plates separated by 20 cm that have a potential difference of 140 V?
- A 100 V battery is connected to two parallel metal plates that are 5.0 cm apart.
- What electric field is generated between these plates?
- What force would a charge of 10−8 C experience in this field?
- What would be the change in energy of this charge if it moved from one plate to the other?
- A particle of charge –2.0 × 10−9 C experiences a force of 10−4 N when between 2 parallel charged plates that are separated by a distance of 4.0 cm.
- What is the strength of the electric field between these plates?
- What is the potential difference across these plates?
- What potential difference must be applied across two parallel plates (15 cm apart) to generate an electric field of 600 V/m?
- What is the force acting on an object having a charge of 10−10 C in this field?
- What is the gain in kinetic energy of this object if it accelerates through a distance of 5.0 cm?
- A particle with a charge of 10−12 C is directed into a 500 N/C electric field.
- What is the force on this particle?
- What is change in its energy after it accelerates through a distance of 1.0 cm?
- What potential difference is needed to accelerate an electron to a speed of 107 m/s?
21.3 Energy, Momentum & Charged Particles
Equations Introduced or Used for this Section:
- [latex]\vec{J}=\Delta\vec{q}[/latex] where [latex]\vec{F}=m\Delta\vec{v}[/latex]
- [latex]E_k=\dfrac{1}{2}mv^2[/latex]
- [latex]\Delta \text{Energy}=\vec{F}\vec{d}\text{ cos ø}[/latex]
- [latex]\vec{F}_{el}=q\vec{E}[/latex]
In this section, we continue to explore the dynamics of charged particles moving through constant electric fields as changes in both their momentum and/or energy. The technological discoveries that resulted from applying the physics developed using a constant electric field are significant. Some of these are: the very first particle accelerators, the first radar and TV tubes, and evidence allowing for the measure of the charge and mass of both the electron and proton. Using the relationship between the change in kinetic energy and the energy change of charged particles inside uniform electric fields also helped in the development of solar panels for electricity and was critical to understanding the relationship between light, energy and charged particles. While deeper inspections into these relationships is only alluded to in this topic, it will be in the study of magnetism when combined with constant electric fields that the major discoveries that were first in providing scientists with a foundational understanding on the structural nature of atoms and molecules.
This section piggybacks on the original change in momentum and energy equations you encountered prior in Chapters 8, 9, 10 and 11: F t = m∆v and Fd cos ø = ∆ Energy. In most ways it is a review and extension of momentum and mechanical energy. The major difference is that now charged particles are affected by electric force and fields and the energy of these accelerated charged particles are defined by the voltage or potential difference they are accelerated or decelerated through. It is also the first Chapter where one pushes up against the limits of classical physics as it is possible with the apparatus and technologies used in this section to require the need of relativistic solutions to be able to understand and produce reliable results.
You will also notice that vector equations are freely used in scalar settings as direction is not needed to arrive at the desired solution. As a result, one can see the above equations written and used as the following:
- [latex]J=\Delta p\text{ where }F\,t=m\Delta v[/latex]
- [latex]E_k =\dfrac{1}{2}mv^2[/latex]
- [latex]\Delta \text{Energy}=\vec{F}\vec{d}\text{ cos ø}[/latex]
- [latex]\vec{F}_{el}=q\vec{E}[/latex]
This exploration of topics is important in your studies as it provides valuable insights that will be built upon in higher levels of physics in your studies of Atomic and Plasma physics. Please enjoy the fact that this is only the start of exploring some of the great adventures and discoveries that brought Classical Physics into the modern world of Relativistic and Quantum Physics.
Example 21.3.1
What is the change in momentum of an electron (starting from rest) that experiences an electric force of 10−15 N for 10−10 s?
Solution
Since no direction is given, we can solve this problem as a scalar
Use Impulse…
- [latex]∆p = F t[/latex]
- [latex]\Delta p[/latex] = (1 × 10−15 N)(1 × 10−10 s)
- [latex]\Delta p[/latex] = 1 × 10−25 Ns or 10−25 Ns
Example 21.3.2
What energy is gained by an electron (starting from rest) that experiences an electric force of 10−15 N for a time span of 10−10 s?
Solution
Since no direction is given, we can solve this problem as a scalar
First… Use Impulse = Change in Momentum
- [latex]F\,t = m∆v[/latex]
- (1 × 10−15 N)(1 × 10−10 s) = (9.11 × 10−31 kg)[latex]\Delta v[/latex]
- [latex]\Delta v[/latex] = (1 × 10−15 N)(1 × 10−10 s) ÷ (9.11 × 10−31 kg)
- [latex]\Delta v[/latex] = 1.1 × 105 m/s
Now… Find the change in kinetic energy
- [latex]∆E_k=E_{kf} −E_{ki}[/latex]… Where [latex]E_{ki} = 0\text{ J}[/latex]
- [latex]∆E_k= \dfrac{1}{2}mvf^2[/latex]
- [latex]E_k=\dfrac{1}{2} (9.11\times 10^{−31}\text{ kg})(1.1 \times 10^5\text{ m/s})^2[/latex]
- [latex]E_k =5.5 \times 10^{−21}\text{ J}[/latex]
Example 21.3.3
What distance must an electron travel in an 10 000 N/C electric field to accelerate from 0.00 c to 0.05 c? (c = 3.00 × 108 m/s)
Solution
First… Find the kinetic energies of an electron traveling at these two speeds…
For 0.00 c…
- [latex]v_{0.00 \ c}[/latex] = 0 m/s
Therefore [latex]Ek[/latex] at 0.00 c = 0 J
For 0.05 c…
- [latex]v_{0.05 \ c}[/latex] = (0.05)(3.00 × 108 m/s)
- [latex]v_{0.05 \ c}[/latex] = 1.5 × 107 m/s
Now… [latex]E_k = \dfrac{1}{2} mv^2[/latex]
- [latex]E_k =\dfrac{1}{2}(9.11\times 10^{−31}\text{ kg})(1.5\times 10^7\text{ m/s})^2[/latex]
- [latex]E_k[/latex] = 1.02 × 10−16 J
Equating the Change in Energy using Net Force acting through some displacement and Kinetic Energy, we equate…
- [latex]\Delta E\vec{F}\vec{d}=q\vec{E}\vec{d}[/latex] and [latex]∆E_k=E_{kf}−E_{ki}[/latex] (Note: [latex]E_{ki}[/latex] = 0 J)
- [latex]q\vec{E}\vec{d} = E_{kf}− E_{ki}[/latex] reduces to [latex]q\vec{E}\vec{d}=E_{kf}[/latex]
This yields
- (1.602 × 10−19 C)(10 000 N/C) [latex]d[/latex] = 1.02 × 10−16 J
- [latex]d[/latex] = 1.02 × 10−16 J ÷ (1.602 × 10−19 C)(10000 N/C)
- [latex]d[/latex] = 6.4 × 10−2 m or 6.4 cm
Exercise 21.3
- What is the change in momentum of an electron (starting from rest) that experiences an electric force of 10−12 N for 10−12 s?
- What energy is gained by an electron (starting from rest) that experiences an electric force of 10−12 N for a time span of 10−12 s?
- What distance must an electron travel in a 5000 N/C electric field to accelerate from 0.00 c to 0.10 c?
- What is the change in momentum of an electron that goes from 0.10 c North to 0.10 c South?
- What is the strength of the electric force that could do this in 1.0 µs?
- What distance would a 0.05 c proton travel into a constant electric field of 50 000 N/C to bring it to a full stop?
- What is the speed that a 100 eV electron travels at?
- What is the kinetic energy of a proton that has the same speed? (answer in eV)
- An electron is suddenly placed into a constant electric field of 4000 N/C.
- What distance does it have to travel to gain a kinetic energy of 50 eV?
- Over what distance would a proton (under the same circumstances as the electron above) have to travel?
- What are the momenta of a
- 200 eV electron?
- 200 eV proton?
- An electron is in a particle accelerator and is accelerated from rest for about 2.5 m in an electric field of an average strength of 100 million N/C. What final speed does Newtonian mechanics predict?
- How far would the electron in problem #8 have to travel to reach 1/10 c? (c is the symbol for the speed of light: 3.00 × 108 m/s. At 1/10 c, Einsteinian corrections are generally done)
Exercise 21.4.1: The Millikan Oil Drop Experiment
The history of the discovery of the proton and electron and later the charge and mass of each particle culminated in the work of Robert A. Millikan and Harvey Fletcher in 1909 and is now known as the Millikan Oil Drop Experiment. Starting in 1897, J. J. Thompson was able to measure the charge to mass ratio of the electron by comparing the deflection of electron beams (then called cathode rays) by electric and magnetic fields. (centripetal force for the electrical field and Newton’s second law for the magnetic field). While Thompson’s work on the electron led him to measure the charge to mass ratio of the electron was 1.3 × 1011 C/kg a measure that has since been refined to 1.7588192(53) × 1011 C/kg. Charge to mass ratios for positive ions were complicated by the protons being part of the nucleus and were multiples of the proton’s mass.
Millikan’s discovery of the charge of the electron allowed scientists to then be able to calculate the masses of the charged particles and hence the mass of the electron, proton, alpha particle and other charged particles. Millikan’s measure was remarkably close to the current value of the electron charge, finding a value of 1.592 × 10−19 C
Question: Using Thompson’s q/m ratio of 1.3 × 1011 C/kg and Millikan’s measure of charge to be 1.592 × 10−19 C. What was Millikan’s first measure of the mass of the electron?
Exercise 21.4.2: An Early Cathode Ray Tube (CRT)
One of the earliest applications of uniform electric fields was in the design and development of Cathode Ray Tubes that were used in the early televisions, oscilloscopes, x-ray tubes and so on. The image shown below represents the standard form of the CAT II Oscilloscope that had a uniform electric field accelerating the electron beam (this is called the electron gun) through a potential difference of 1 kV. Once the beam left the electric field it was then deflected using electric statically charged plates to deflect the beam onto a screen that would then glow from the collision of the 1 keV electron.
These tubes had to be designed and operated in a glass vacuum. The nature of this device required the electron beam to be deflected at very high speeds.
Question: If the distance from the end of electron gun to the fluorescent screen is 20 cm, what was the expected time of flight for this duration?
Exercise 21.4.3: Relativistic Charged Particles
As “charged” particle accelerators increase the potential difference charged particles are accelerated through, corrections must be made in how time, mass and length are measured. The concept of the measure of mass increases is one that is under review from physics educators since it promotes the notion that mass increases rather than the dimensions of space and time are altered (You will need to wait for these answers in a few more years). Nevertheless, there is a useful equation that can be used to correct for errors that will occur when observing high energy particles. In fact, the first confirmation Einstein’s Special Relativity occurred in 1908 by trying to deflect high energy electrons in a vacuum tube similar to the one described in 18.4.2
Question: If an electron is accelerated through a potential difference of 1.0 MV, what would be the measured speed of this electron? Have fun:)
Use the equation… [latex]\dfrac{mc^2}{\sqrt{1-(v/c)^2}}=E_k+mc^2[/latex]
Exercise Answers
21.1 Energy Change of Charge moving through a Uniform Electric Field
- 3.5 × 108 J
- 0.225 J
- 2.4 × 10−15 J or 15000 eV
- 5000 eV or 5 keV
21.2 Uniform Electric Fields (Parallel Charged Plates)
- 0.04 m
- 630 V
- 700 N/C
- 2000 N/C
- 2.0 × 10−5 N
- 10−6 J or 1 µJ
- 50000 N/C
- 2000 V
- 90 V
- 6.0 × 10−8 N
- 3.0 × 10−9 J
- 5.0 × 10−10 N
- 5.0 × 10−12 J
- 285 V (≈ 300 V)
21.3 Energy, Momentum & Charged Particles
- 10−24 Ns
- 5.49 × 10−19 J or 3.43 eV
- 0.51 m
- 5.47 × 10−23 Ns South
- 5.47 × 10−17 N
- 23.5 m
- 5.93 × 106 m/s
- 183 000 eV or 0.183 MeV
- 0.0125 m
- 0.0125 m
- 7.64 × 10−24 Ns
- 3.27 × 10−22 Ns
- 9.4 × 109 m/s… around 31 time the speed of light (≈ 31 c)
- 2.56 × 10−5 m (or 25.6 µm)
21.4.1 The Millikan Oil Drop Experiment
- First estimated mass of the electron… me- = 1.22 × 10−30 kg
21.4.2 An Early Cathode Ray Tube (CRT)
- t = 1.1 × 10−8 s or 11 ns
21.4.3 Relativistic Charged Particles
- v = 2.44 × 108 m/s or 0.81c

