Principles of Pharmacology
1.2 Pharmacokinetics and Pharmacodynamics
Pharmacokinetics
Pharmacokinetics is the term that describes the four stages of absorption, distribution, metabolism, and excretion of drugs. Drugs are medications or other substances that have a physiological effect when introduced to the body. There are four basic stages for a medication to go through within the human body: absorption, distribution, metabolism, and excretion. This entire process is sometimes abbreviated ADME. Absorption occurs after medications enter the body and travel from the site of administration into the body’s circulation. Distribution is the process by which medication is distributed throughout the body. Metabolism is the breakdown of a drug molecule. Excretion is the process by which the body eliminates waste. Each of these stages is described separately later in this chapter.
Research scientists who specialize in pharmacokinetics must also pay attention to another dimension of drug action within the body: time. Unfortunately, scientists do not have the ability to actually see where a drug is going or how long it is active. To compensate, they use mathematical models and precise measurements of blood and urine to determine where a drug goes and how much of the drug (or breakdown product) remains after the body processes it. Other indicators, such as blood levels of liver enzymes, can help predict how much of a drug is going to be absorbed.
Principles of chemistry are also applied while studying pharmacokinetics because the interactions between drug and body molecules are really just a series of chemical reactions. Understanding the chemical encounters between drugs and biological environments, such as the bloodstream and the oily surfaces of cells, is necessary to predict how much of a drug will be metabolized by the body.
Pharmacodynamics
Pharmacodynamics refers to the effects of drugs in the body and the mechanism of their action. As a drug travels through the bloodstream, it will exhibit a unique affinity for the drug-receptor site, meaning how strongly it will bind to the site. Other components of pharmacodynamics include ion channels, enzymes, and the immune system.
Examination of the ways in which drugs and receptor sites create a lock and key system (see Figure 1.2a[1]) is helpful to understand how drugs work and the amount of drug that may be left circulating within the bloodstream. This concept is broadly termed as drug bioavailability. The bioavailability of drugs is an important feature that chemists and pharmaceutical scientists keep in mind when designing and packaging medicines.
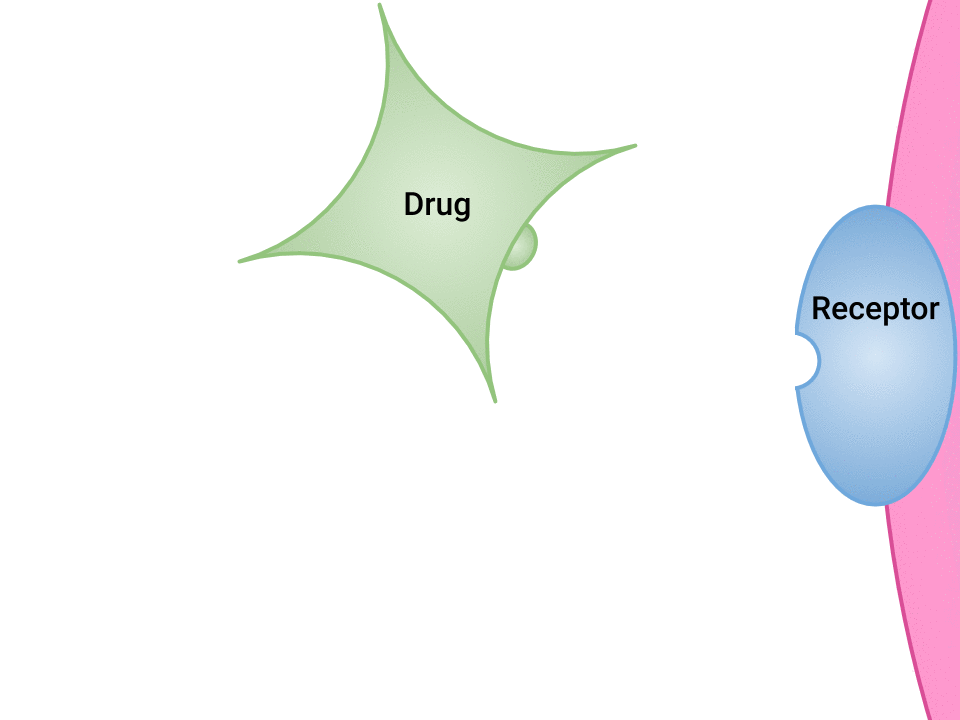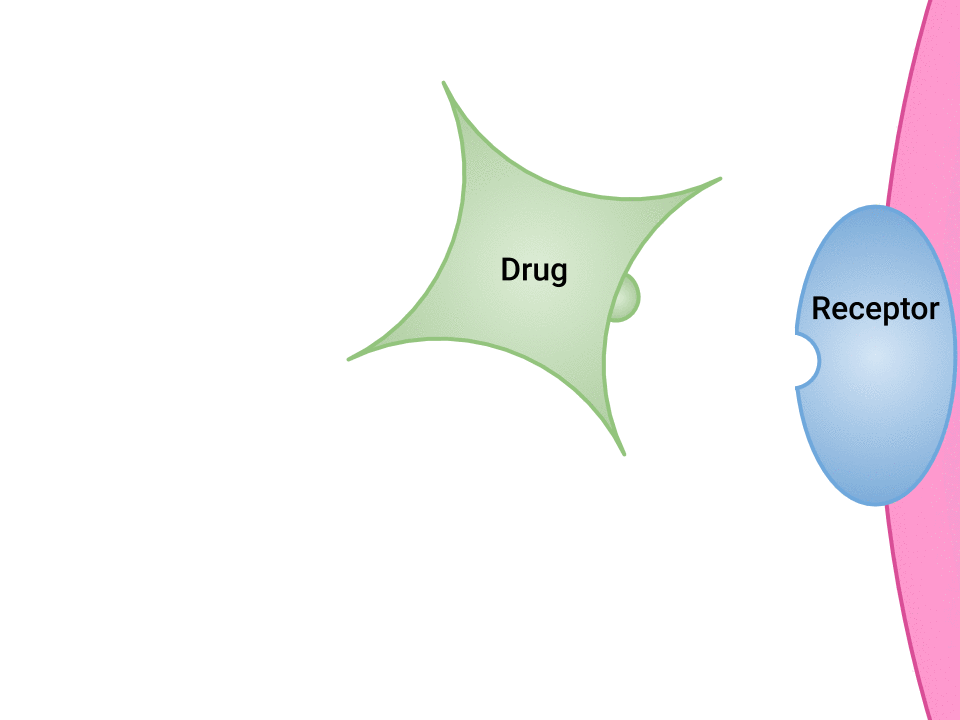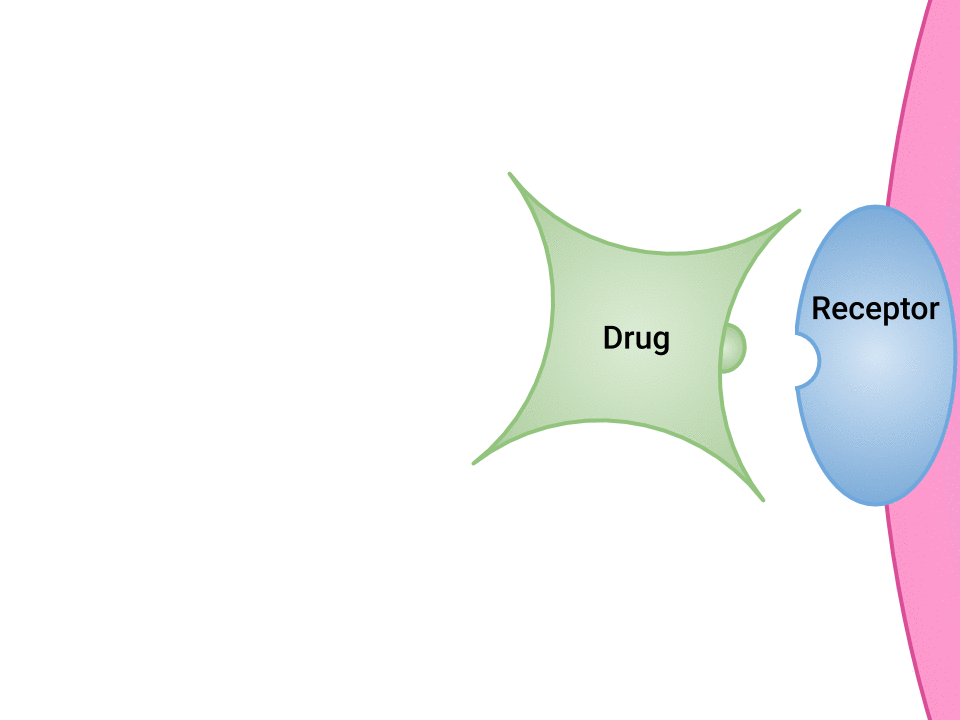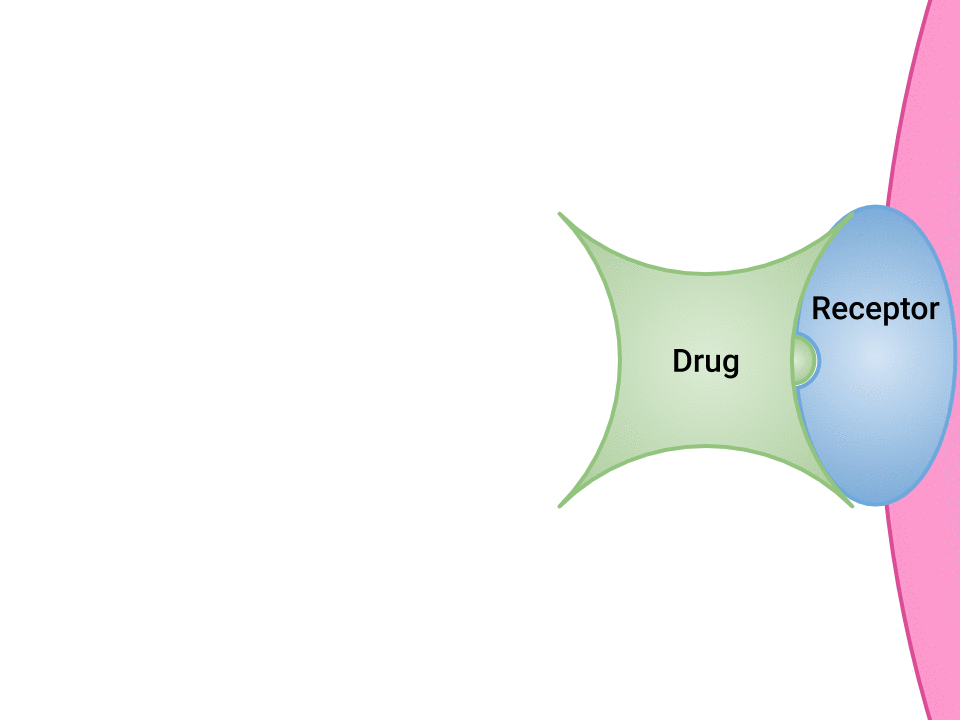
The Relationship Between Pharmacokinetics and Pharmacodynamics
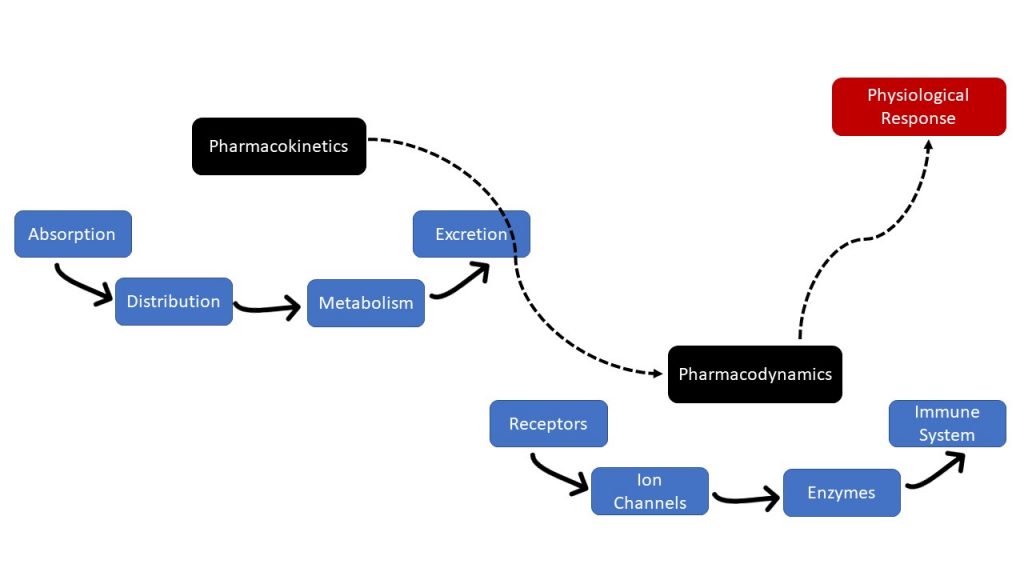
Essentially, pharmacokinetics is the movement of drugs through the body, and pharmacodynamics is the body’s biological response to the drugs. Pharmacokinetics and pharmacodynamics need to be considered when administering medications. Figure 1.2b displays the relationship between pharmacokinetics and pharmacodynamics.
Pharmacogenetics
No matter how effectively a drug works in a laboratory simulation, the performance in the human body will not always produce exactly the same results, and individualized responses to drugs have to be considered. Although many responses to medications may be anticipated, one’s unique genetic makeup may also have a significant impact on one’s response to a drug. Pharmacogenetics is defined as the study of how people’s genes affect their response to medicines.[2].
Image Description
Figure 1.2b image description:
- Pharmacokinetics
- Absorption
- Distribution
- Metabolism
- Excretion
- Pharmacodynamics
- Receptors
- Ion Channels
- Enzymes
- Immune System
- Physiological Response [Return to Figure 1.2b]
- "Drug and Receptor Binding" by Dominic Slausen at Chippewa Valley Technical College is licensed under CC BY 4.0 ↵
- Davis, A. (2006). Medicines by design. U.S. Department of Health and Human Services. https://nigms.nih.gov/education/Booklets/medicines-by-design ↵
The study of how the body absorbs, distributes, metabolizes, and eliminates drugs.
Medications or other substances that have a physiological effect when introduced to the body.
The first stage of pharmacokinetics: medications enter the body and travel from the site of administration into the body's circulation.
The second stage of pharmacokinetics; the process by which medication is distributed throughout the body.
The breakdown of a drug molecule via enzymes in the liver (primarily) or intestines (secondarily).
The final stage of pharmacokinetics; process by which the body eliminates waste or excess.
The study of how drugs act at target sites of action in the body.
The strength of binding between drug and receptor.
The ability of a drug or other chemical to be taken up by the body and made available in the tissue where it is needed.
The study of how people's genes affect their response to medicines.

