Chapter 16. Organic Chemistry
End-of-Chapter Material
Additional Exercises
- Cycloalkanes are named based on the number of C atoms in them, just like regular alkanes, but with the prefix cyclo– on the name. What are the names of the three smallest cycloalkanes?
- Cycloalkenes are named similarly to cycloalkanes (see Exercise 1). What are the names of the cycloalkenes with five, six, and seven C atoms?
- Draw the bond-line structure of all noncyclic alkanes with only four C atoms.
- Draw the bond-line structure of all noncyclic alkanes with only five C atoms.
- Cyclic alkanes can also have substituent groups on the ring. Draw the bond-line structure of all cyclic alkanes with only four C atoms.
- Cyclic alkanes can also have substituent groups on the ring. Draw the bond-line structure of all cyclic alkanes with only five C atoms.
- Draw and name all possible isomers of pentene.
- Draw and name all possible normal (that is, straight-chain) isomers of heptyne.
- Polyunsaturated alkenes have more than one C–C double bond. Draw the carbon backbone of all possible noncyclic polyunsaturated alkenes with four C atoms and two double bonds. What are the complete molecular formulas for each possible molecule?
- Draw the carbon backbone of all possible five-carbon cyclic alkenes with two double bonds, assuming no substituents on the ring.
- If a hydrocarbon is combined with enough halogen, all the H atoms will eventually be substituted with that halogen atom. Write the balanced chemical reaction between ethane and excess chlorine.
- If a hydrocarbon is combined with enough halogen, all the H atoms will eventually be substituted with that halogen atom. Write the balanced chemical reaction between butane and excess bromine.
- Molecules with multiple double bonds can also participate in addition reactions. Draw the structure of the product when butadiene, CH2=CH–CH=CH2, reacts with chlorine.
- Draw the structure of the product when allene, CH2=C=CH2, reacts with bromine.
- What is the maximum number of methyl groups that can be on a propane backbone before the molecule cannot be named as a propane compound?
- Explain why cycloethane cannot exist as a real molecule.
- In the gasoline industry, what is called isooctane is actually 2,2,4-trimethylpentane. Draw the structure of isooctane.
- Isooctane (see Exercise 17) is an isomer of what straight-chain alkane?
- The actual name for the explosive TNT is 2,4,6-trinitrotoluene. If the structure of TNT is as shown below, propose the structure of the parent compound toluene.
![Rendered by QuickLaTeX.com \chemfig{-[:-90]*6(-(-O_2N)=-(-NO_2)=-(-NO_2)=)}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-5f3b5315825853f39be421df469529f4_l3.png)
- Phenol is hydroxybenzene, the simplest aromatic alcohol. Picric acid is an explosive derivative of phenol whose formal name is 2,4,6-trinitrophenol. With reference to Exercise 19, draw the structure of picric acid.
- Draw the structures of all possible straight-chain isomers of bromopentane.
- Draw the structures of all the possible isomers of butanol. Include branched isomers.
- What is the final product of the double elimination of HCl from 1,1-dichloroethane?
- Draw the structure of the final product of the double elimination of 1,3-dibromopropane.
- Draw the structure of and name the alcohol whose double elimination would yield the same product as in Exercise 23. Name the molecule as a hydroxyl-substituted compound.
- Draw the structure of and name the alcohol whose double elimination would yield the same product as in Exercise 24. Name the molecule as a hydroxyl-substituted compound.
- Draw the smallest molecule that can have a separate aldehyde and carboxylic acid group.
- Name the functional group(s) in the following structure:
![Rendered by QuickLaTeX.com \chemfig{*6(-=(*5(-(--[:0]NH_2)--(=O)-))-=-(-HO)=)}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-2b15678d467f873f5c5644fff4c09731_l3.png)
- Ethyl acetate is a common ingredient in nail-polish remover because it is a good solvent. Draw the structure of ethyl acetate.
- A lactone is an ester that has its ester functional group in a ring. Draw the structure of the smallest possible lactone. (It is called acetolactone, which might give you a hint about its structure.)
- Draw the structure of diethyl ether, once used as an anaesthetic.
- The smallest cyclic ether is called an epoxide. Draw its structure.
- Write the chemical reaction of HCl with trimethylamine.
- Putrescine and cadaverine are molecules with two amine groups on the opposite ends of a butane backbone and a pentane backbone, respectively. They are both emitted by rotting corpses. Draw their structures and determine their molecular formulas.
- With four monomers, draw two possible structures of a copolymer composed of ethylene and propylene.
- With four monomers, draw two possible structures of a copolymer composed of ethylene and styrene.
- Draw the silicone that can be made from this monomer:
![Rendered by QuickLaTeX.com \chemfig{[:90]*6((-[:-90]Si?[a]-[:-90])-----(-[:-90]Si?[a,2]-[:-90]([:-60]*6(-----))))}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-19c94e3fea9554656932da0f664e5220_l3.png)
- One of the ingredients in the original Silly Putty was a silicone polymer with two methyl groups on each Si atom. Draw this silicone.
Answers
- cyclopropane, cyclobutane, and cyclopentane
- Bond-line structure of all noncyclic alkanes with only four C atoms:
![Rendered by QuickLaTeX.com \chemfig{-[:-30]-[:30]-[:-30]}\hspace{4em}\chemfig{-[:-90](-[:-150])-[:-30]}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-9edbbda6b01212dea3261d8b4862d726_l3.png)
- Bond-line structure of all cyclic alkanes with only four C atoms:
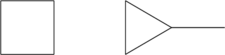
- Isomers of pentene:
![Rendered by QuickLaTeX.com \begin{align*} \chemfig{=[:30]-[:-30]-[:30]-[:-30]}&\hspace{4em}\chemfig{-[:30]=[:-30]-[:30]-[:-30]} \\ \\ \chemfig{=[:30](-[:90])-[:-30]-[:30]}&\hspace{4em}\chemfig{-[:30](-[:90])=[:-30]-[:30]} \end{align*}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-fa9b4e9f05b6d4a1c1c2a7869d0d2353_l3.png)
- Carbon backbone of all possible noncyclic polyunsaturated alkenes with four C atoms and two double bonds:
![Rendered by QuickLaTeX.com \chemfig{H_2C=C=CH-[:-60]CH_3}\hspace{4em}\chemfig{H_2C=[:-60]HC-CH=[:-60]CH_2}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-de99584fb2313b482b9fde7c1378ad7b_l3.png)
Both molecular formulas are C4H6.
- C2H6 + 6Cl2 → C2Cl6 + 6HCl
- Structure of butadiene’s reaction with chlorine:
![Rendered by QuickLaTeX.com \chemfig{H-(-[:90]Cl)(-[:-90]H)-(-[:90]Cl)(-[:-90]H)-(-[:90]Cl)(-[:-90]H)-(-[:90]Cl)(-[:-90]H)-H}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-3561ef3fa7956f1f5007cbfb282cd166_l3.png)
- two
- Structure of isooctane:
(-[:150])-[:-30](-[:-90])-[:30]-[:-30]}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-2ee1c184b47259376d67d506b32688de_l3.png)
- Structure of toluene:
![Rendered by QuickLaTeX.com \chemfig{-[:-90]*6(-=-=-=)}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-f4359d29688a62125e0a564937f2fb58_l3.png)
- Straight-chain isomers of bromopentane:
![Rendered by QuickLaTeX.com \chemfig{Br-[:30]-[:-30]-[:30]-[:-30]-[:30]}\hspace{4em}\chemfig{-[:30](-[:90]Br)-[:-30]-[:30]-[:-30]}\hspace{4em}\chemfig{-[:-30]-[:30](-[:90]Br)-[:-30]-[:30]}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-1bad72c468d64181e7cc13f6e6ddb726_l3.png)
- ethyne
![Rendered by QuickLaTeX.com \begin{align*}\chemfig{HO-[:-60]--[:60]OH} &\hspace{2em} \chemfig{HO-[:-60](-[:-120]HO)-}\end{align*}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-cd7aa6a94aa0d6bca549655749011fe2_l3.png)
- The names are 1,2-dihydroxyethane and 1,1-dihydroxyethane, respectively.
- (CH3)3N + HCl → (CH3)3NHCl
- (answers will vary)
![Rendered by QuickLaTeX.com \begin{array}{c}\chemfig{-[@{left,0.5}]C(-[:90]H_2)-C(-[:90]H_2)-C(-[:90]H)(-[:-90]CH_3)-C(-[:90]H_2)-C(-[:90]H_2)-C(-[:90]H_2)-C(-[:90]H)(-[:-90]CH_3)-C(-[:90]H_2)-[@{right,0.5}]} \polymerdelim[delimiters={[]},height=25 pt]{left}{right} \\ \\ \chemfig{-[@{left,0.5}]C(-[:90]H_2)-C(-[:90]H)(-[:-90]CH_3)-C(-[:90]H_2)-C(-[:90]H_2)-C(-[:90]H_2)-C(-[:90]H_2)-C(-[:90]H)(-[:-90]CH_3)-C(-[:90]H_2)-[@{right,0.5}]} \polymerdelim[delimiters={[]},height=25 pt]{left}{right} \end{array}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-0b0c006dde18bee327d80c06fb707f20_l3.png)

-[:30](=[:90]O)-[:-30]OH}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-e1ba3facc8602e357e7954592ac5d78c_l3.png)
-[:-30]O-[:30]-[:-30]}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-7bcd98d2c68c80fb4a05aea506df688c_l3.png)
![Rendered by QuickLaTeX.com \chemfig{-[@{left,0.5}]Si(-[:90])(-[:-90]([:-60]*6(-----)))-Si(-[:90]([:120]*6(-----)))(-[:-90])-[:0, 2.0]Si(-[:90])(-[:-90]([:-60]*6(-----)))-Si(-[:90]([:120]*6(-----)))(-[:-90])-[@{right,0.5}]} \polymerdelim[delimiters={[]},height=20 pt]{left}{right}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-98f095d91101c6bde85b0dc351bd7cb9_l3.png)
