Chapter 9. Chemical Bonds
End-of-Chapter Material
Additional Exercises
- Explain why iron and copper have the same Lewis electron dot diagram when they have different numbers of electrons.
- Name two ions with the same Lewis electron dot diagram as the Cl− ion.
- Based on the known trends, what ionic compound from the first column of the periodic table and the next-to-last column of the periodic table should have the highest lattice energy?
- Based on the known trends, what ionic compound from the first column of the periodic table and the next-to-last column of the periodic table should have the lowest lattice energy?
- P2 is not a stable form of phosphorus, but if it were, what would be its likely Lewis electron dot diagram?
- Se2 is not a stable form of selenium, but if it were, what would be its likely Lewis electron dot diagram?
- What are the Lewis electron dot diagrams of SO2, SO3, and SO42−?
- What are the Lewis electron dot diagrams of PO33− and PO43−?
- Which bond do you expect to be more polar: an O–H bond or an N–H bond?
- Which bond do you expect to be more polar: an O–F bond or an S–O bond?
- Use bond energies to estimate the energy change of this reaction.
C3H8 + 5O2 → 3CO2 + 4H2O
- Use bond energies to estimate the energy change of this reaction.
N2H4 + O2 → N2 + 2H2O
- Ethylene (C2H4) has two central atoms. Determine the geometry around each central atom and the shape of the overall molecule.
- Hydrogen peroxide (H2O2) has two central atoms. Determine the geometry around each central atom and the shape of the overall molecule.
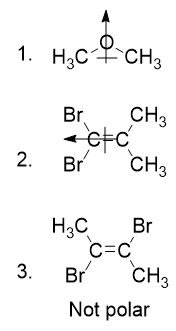
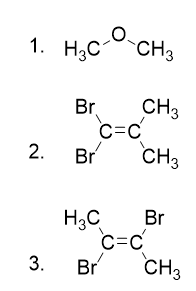
- Determine the molecular dipole moments for the following molecules:
- What is the hybridization in the central atom of:
- HCN
- NH4
- TeBr2
- Draw the molecular orbital electron configuration energy diagram of He2 and determine the bond order.
- Draw the molecular orbital electron configuration energy diagram of O2 and determine the bond order.
Answers
- Iron has d electrons that typically are not shown on Lewis electron dot diagrams.
- LiF
- It would be like N2:

- an O–H bond
- −2,000 kJ
- trigonal planar about both central C atoms
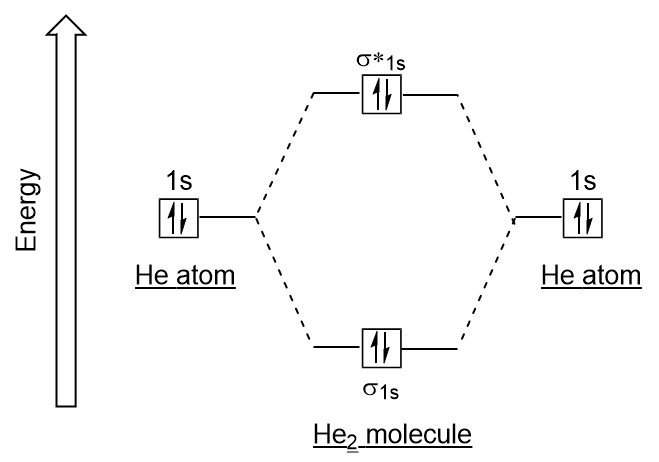
bond order = 0

![Rendered by QuickLaTeX.com \chemfig{\Lewis{2:4:,O}=\Lewis{6:,S}=\Lewis{0:6:,O}}, \chemfig{\Lewis{4:6:,O}=[:30]S(=[:90]\Lewis{0:2:,O})=[:-30]\Lewis{0:6:,O}},\text{ and }\chemfig{\Lewis{2:4:6:,O}-\ce{S^{2-}}(=[:90]\Lewis{0:4:,O})(=[:-90]\Lewis{0:4:,O})-\Lewis{0:2:6:,O}}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-441905c80e51c2b34817ea7ea4b7fea5_l3.png)