Chapter 1 Introduction to Geology
1.4 Minerals and Rocks
The rest of this chapter is devoted to a brief overview of a few of the important aspects of physical geology, starting with minerals and rocks. This is followed by a review of Earth’s internal structure and the processes of plate tectonics, and an explanation of geological time.
The Earth is made up of varying proportions of the 90 naturally occurring elements—hydrogen, carbon, oxygen, magnesium, silicon, iron, and so on. In most geological materials, these combine in various ways to make minerals. Minerals will be covered in some detail in Chapter 2, but here we will briefly touch on what minerals are, and how they are related to rocks.
A mineral is a naturally occurring combination of specific elements that are arranged in a particular repeating three-dimensional structure or lattice.[1] The mineral halite is shown as an example in Figure 1.4.1.
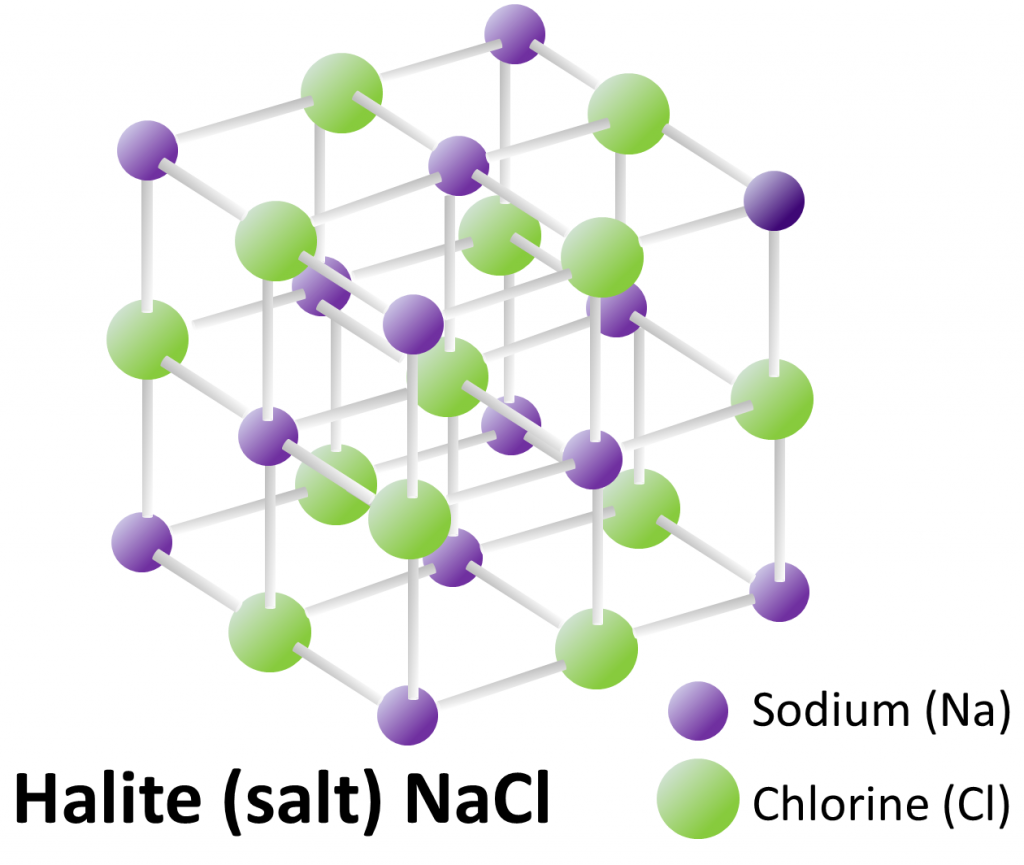
In this case, atoms of sodium (Na: purple) alternate with atoms of chlorine (Cl: green) in all three dimensions, and the angles between the bonds are all 90°. Even in a tiny crystal, like the ones in your salt shaker, the lattices extend in all three directions for thousands of repetitions. Halite always has this composition and this structure.
| Note: Element symbols (e.g., Na and Cl) are used extensively in this book. In Appendix 1, you will find a list of the symbols and names of the elements common in minerals and a copy of the periodic table. Please use those resources if you are not familiar with the element symbols. |
There are thousands of minerals, although only a few dozen are mentioned in this book. In nature, minerals are found in rocks, and the vast majority of rocks are composed of at least a few different minerals. A close-up view of granite, a common rock, is shown in Figure 1.4.2. Although a hand-sized piece of granite may have thousands of individual mineral crystals in it, there are typically only a few different minerals, as shown here.

Rocks can form in a variety of ways. Igneous rocks form from magma (molten rock) that has either cooled slowly underground (e.g., to produce granite) or cooled quickly at the surface after a volcanic eruption (e.g., basalt). Sedimentary rocks, such as sandstone, form when the weathered products of other rocks accumulate at the surface and are then buried by other sediments. Metamorphic rocks form when either igneous or sedimentary rocks are heated and squeezed to the point where some of their minerals are unstable and new minerals form to create a different type of rock. An example is schist.
A critical point to remember is the difference between a mineral and a rock. A mineral is a pure substance with a specific composition and structure, while a rock is typically a mixture of several different minerals (although a few types of rock may include only one type of mineral). Examples of minerals are feldspar, quartz, mica, halite, calcite, and amphibole. Examples of rocks are granite, basalt, sandstone, limestone, and schist.
Key Takeaway: Know the difference between minerals and rocks!
If you are currently taking a geology course, you’ll likely be asked more than once to name a mineral or a rock that has specific characteristics or composition, or was formed in a specific environment. Please make sure that if you’re asked for a rock name that you don’t respond with a mineral name, and vice versa. Confusing minerals and rocks is one of the most common mistakes that geology students make.
Exercise 1.1 Find a piece of granite
The rock granite is very common in most parts of North America, and unless everything is currently covered with snow where you live, you should have no trouble finding a sample of it near you. The best places to look are pebbly ocean or lake beaches, a gravel bar of a creek or river, a gravel driveway, or somewhere where gravel has been used in landscaping. In Figure 1.4.3, taken on a beach, the granitic pebbles are the ones that are predominantly light-coloured with dark specks. The one in the very centre is a good example.

Select a sample of granite and, referring to Figure 1.4.2, see if you can identify some of the minerals in it. It may help to break it in half with a hammer to see a fresh surface, but be careful to protect your eyes if you do so. You should be able to see glassy-looking quartz, dull white plagioclase feldspar (and maybe pink potassium feldspar), and black hornblende or, in some cases, flaky black biotite mica (or both).
In addition to identifying the minerals in your granite, you might also try to describe the texture in terms of the range sizes of the mineral crystals (in millimetres) and the shapes of the crystals (some may be rectangular in outline, most will be irregular). Think about where your granite might have come from and how it got to where you found it.
See Appendix 3 for Exercise 1.1 answers.
Media Attributions
- Figure 1.4.1, 1.4.2, 1.4.3: © Steven Earle. CC BY.
- Terms in bold are defined in the glossary at the end of the book. ↵
The regular and repeating three-dimensional structure of a mineral.
NaCl, a halide mineral also known as table salt.
A felsic intrusive igneous rock.
Molten rock typically dominated by silica.
A volcanic rock of mafic composition.
A rock that is primarily comprised of sand-sized particles.
A metamorphic rock with visible aligned mica crystals.

