19.4 Isotopic Dating Methods
Isotope Pairs
Isotopes of an element have different numbers of neutrons. Sometimes this just makes one of the isotopes slightly lighter or heavier than others, but other times it makes the element unstable, causing it to undergo radioactive decay. Unstable elements paired with the elements they decay to are used in isotopic dating methods.
In most cases, we can’t use isotopic techniques to directly date fossils or the sedimentary rocks in which they are found, but we can constrain their ages by dating igneous rocks that cut across sedimentary rocks, or volcanic ash layers that lie within sedimentary layers.
Isotopic dating of rocks, or the minerals within them, is based upon the fact that we know the decay rates of certain unstable isotopes of elements, and that these decay rates have been constant throughout geological time. It is also based on the premise that when the atoms of an element decay within a mineral or a rock, they remain trapped in the mineral or rock, and don’t escape.
How It Works: Potassium-Argon Dating
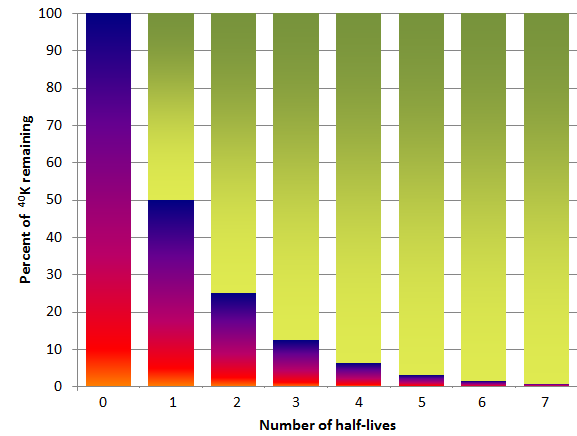
One of the isotope pairs commonly used to date rocks is the decay of 40K to 40Ar (potassium-40 to argon-40). 40K is a radioactive isotope of potassium that is present in very small amounts in all minerals that contain potassium. It has a half-life of 1.3 billion years, meaning that over a period of 1.3 Ga one-half of the 40K atoms in a mineral or rock will decay to 40Ar, and over the next 1.3 Ga one-half of the remaining atoms will decay, and so forth (Figure 19.15). 40K is called the parent isotope, and 40Ar the daughter isotope, as the parent gives way to the daughter during radioactive decay.
Misconception Alert
When we look at radioactive decay, we’re always thinking about half-life as it applies to the remaining atoms of a radioactive isotope, and not necessarily the original amount. In other words, after the second half life, another half of the 50% is remaining (i.e., 25%), rather than all of the remaining 50% being gone. That’s why the dark coloured bars in Figure 19.15 don’t go to zero for the second half-life.
In order to use the K-Ar dating technique, we need to have an igneous or metamorphic rock that includes a potassium-bearing mineral. In granite, we can use the mineral potassium feldspar (salmon-coloured crystals in Figure 19.16). When potassium feldspar forms, it has no argon. But over time, the 40K in the feldspar decays to 40Ar, and the atoms of 40Ar remain embedded within the crystal, unless the rock is subjected to high temperatures after it forms.

The sample must be analyzed using a very sensitive mass-spectrometer, which can detect the differences between the masses of atoms, and can therefore distinguish between 40K and the much more abundant 39K. The minerals biotite and hornblende are also commonly used for K-Ar dating.
Other Isotope Pairs
There are many isotope pairs that can be employed in dating igneous and metamorphic rocks (Table 19.1), each with its strengths and weaknesses. In the above example, the daughter isotope 40Ar is naturally a gas, and can escape the potassium feldspar quite easily if the feldspar is exposed to heating during metamorphism, or interaction with hydrothermal fluids. This means we have to examine the feldspar mineral closely first to see if there is any evidence of alteration. If some 40Ar has been lost, but the sample is dated anyway, the age we get will be too young, because it will look like the argon has been accumulating for less time than it really has.
| Isotope System | Half-life | Useful Range | Comments |
|---|---|---|---|
| Potassium-Argon | 1.3 Ga | 10 Ka – 4.57 Ga | Widely applicable because most rocks contain potassium |
| Uranium-Lead | 4.5 Ga | 1 Ma – 4.57 Ga | The rock must contain uranium-bearing minerals (felsic igneous rocks) |
| Rubidium-Strontium | 47 Ga | 10 Ma – 4.57 Ga | Less precision than other methods for old rocks |
| Carbon-Nitrogen (radiocarbon dating) | 5,730 a | 100 a to 60,000 a | Sample must contain wood, bone, or carbonate minerals; can be applied to young sediments |
Each parent isotope has a certain half-life, which ranges from microseconds to billions of years, depending on the isotope. In dating rocks, we need to select an isotope pair with a parent isotope that has a reasonable half-life for our sample. If the half-life is too short, then most of the parent isotope will have decayed to form the daughter isotope. If we can’t measure the amount of parent isotope very accurately, which will be impossible to do if there is only the tiniest amount of parent isotope left, our calculated age will have huge errors associated with it. The same applies if the half-life is too long. In this case, very little of the daughter isotope will have formed, and our inability to measure the small amount of daughter isotope accurately will again result in huge errors in our calculated age.
Another complicating factor is whether the mineral of interest incorporated any of the daughter isotope into its structure at the time of formation. When we select a mineral and an isotope pair to date that mineral, we make the assumption that all of the daughter isotope we find in the mineral was produced in the mineral by radioactive decay of the parent isotope. But if the mineral formed with some daughter isotope already present in its structure, then the age we calculate will be too old.
A more robust mineral to use to date certain types of igneous and metamorphic rocks is zircon. Zircon is a mineral that incorporates uranium into its structure at the time of formation. One of the isotopes of uranium decays to lead with a long half-life (4.5 Ga). Zircon is a mineral of choice for dating because it takes no lead into its structure when it forms, so any lead present is due entirely to the radioactive decay of the uranium parent. Another reason is because zircon is a very resistant mineral. It can handle exposure to hydrothermal fluids, and all but the highest grades of metamorphism, and not lose any of the parent or daughter isotopes. One drawback is that zircon tends to form only in felsic igneous rocks, so if we’re trying to date a mafic rock, we need to use a different mineral.
The Meaning of a Radiometric Date
When we employ isotopic methods on minerals, we’re measuring an age date. Generally, an age date refers to the time since a mineral crystallized from molten rock, when the elements that make up the mineral got locked into the mineral’s structure. But as we’ve already seen, elevated temperatures can cause elements to escape from a mineral, without the mineral melting. This means that when we date a mineral, we might actually be dating the time since the mineral last experienced a period of heating above its Curie point, which is the temperature beyond which the mineral is able to lose (or gain) elements from its structure, without melting.
So we have to know something about the rock before we forge ahead to measure an age. We may choose a mineral and isotope pair that are very resistant to metamorphism, so that we can “see through” the metamorphism, and determine the original age that the mineral crystallized from a melt. Or we may be interested in the age of the metamorphic event itself, so choose a mineral and isotope pair that is susceptible to resetting the isotopic clock during metamorphism (such as by losing all of the daughter isotope).
Absolute age dating is a powerful tool for unraveling the geological history of a region, but we must ultimately rely upon igneous rocks (that may have later metamorphosed) for the minerals that we are able to date (more on issues with dating sedimentary rocks below). Another issue with absolute age dating is that it is expensive, and a single analysis can cost hundreds of dollars. This is why geologists never forget their relative age dating principles, and are always applying them in the field to determine the sequence of events that formed the rocks in a region.
Combining Absolute Ages with Relative Dating
The age dates for three igneous rock layers are given. Can you figure out the age ranges for the sets of sedimentary units A, B, and C?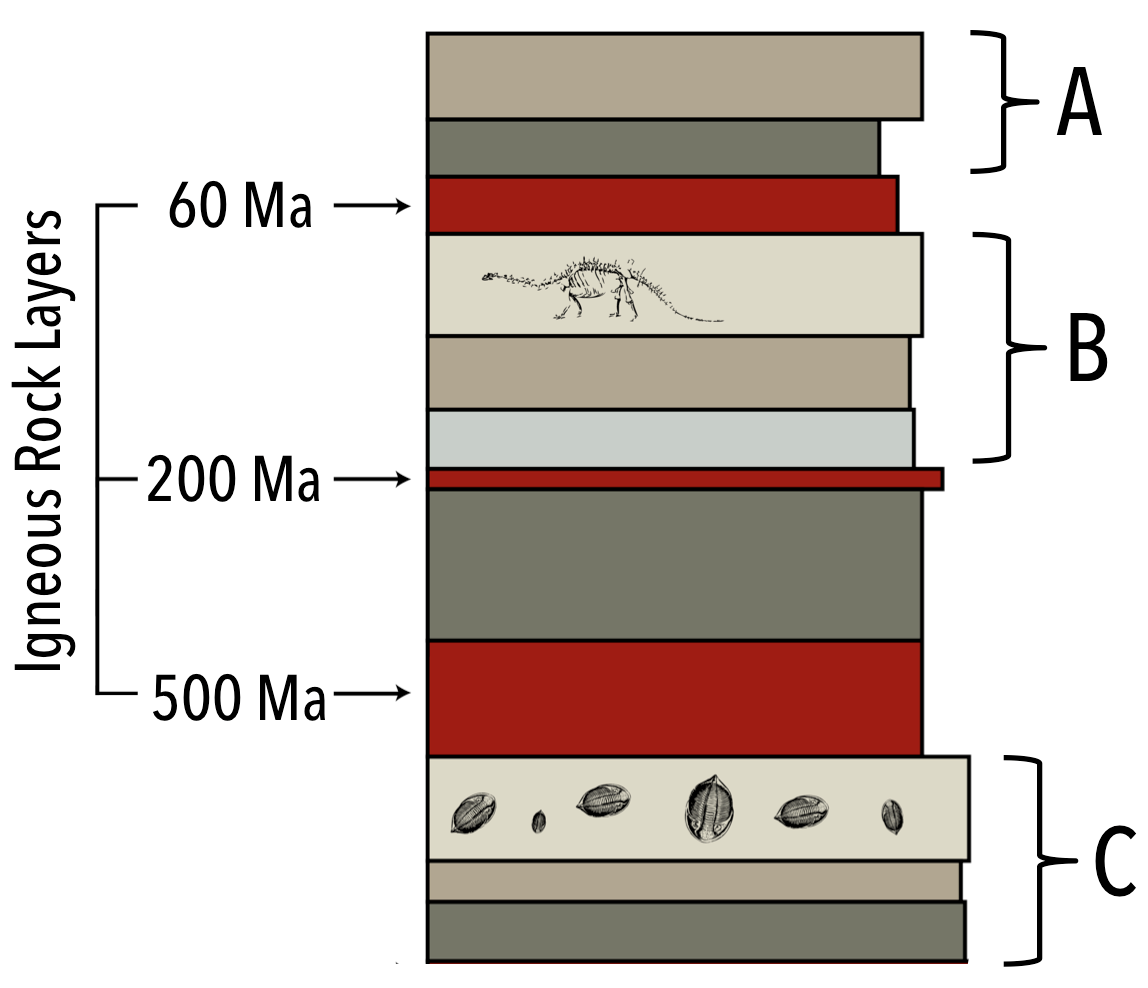
Important first step!
We have to be sure that the igneous rock layers are
so we can use the principle of . If they’re the principle will apply instead, and this won’t work as well.
- cross-cutting
- lava flows
- superposition
- sills
Fill in the ages in the blank spaces below. Let’s get dating?
- The oldest Layer A could be is Ma.
- The youngest Layer A could be is Ma (Note: the stratigraphic column may not be complete).
- The oldest Layer B could be is Ma.
- The youngest Layer B could be is Ma.
- The oldest Layer C could be is Ma.
- The youngest Layer C could be is Ma (Note: This stratigraphic column may not be complete).
Oh dear. It looks like there was a mix-up at the lab, and you got bad info. The igneous layers are sills, not lava flows. Can you salvage this mess and still com eup with some age constraints on the sedimentary rock? When you have the answer, navigate to the link below to see if you’re right.
To check your answers, navigate to the below link to view the interactive version of this activity.
Isotope Dating Techniques and Sedimentary Rocks

A clastic sedimentary rock (e.g., conglomerate, Figure 19.17) is made up of older rock and mineral fragments. These fragments were derived from weathering and erosion of pre-existing rocks. The process of forming a sedimentary rock from sediments generally occurs at low temperatures, so the minerals are not heated beyond their Curie points. This means the minerals still preserve their original ages (either igneous crystallization age, or a metamorphic age), but what does that actually say about the age of the sedimentary rock?
In almost all cases, the fragments have come from a range of source rocks that all formed at different times. If we dated a number of individual grains in the sedimentary rock, we would likely get a range of different dates, all older than the age of the sedimentary rock. The most that such ages gleaned from a sedimentary rock can tell us is a maximum age of the sedimentary rock. It might be possible to date some chemical sedimentary rocks isotopically, but there are no useful isotopes that can be used on old chemical sedimentary rocks.
Radiocarbon Dating
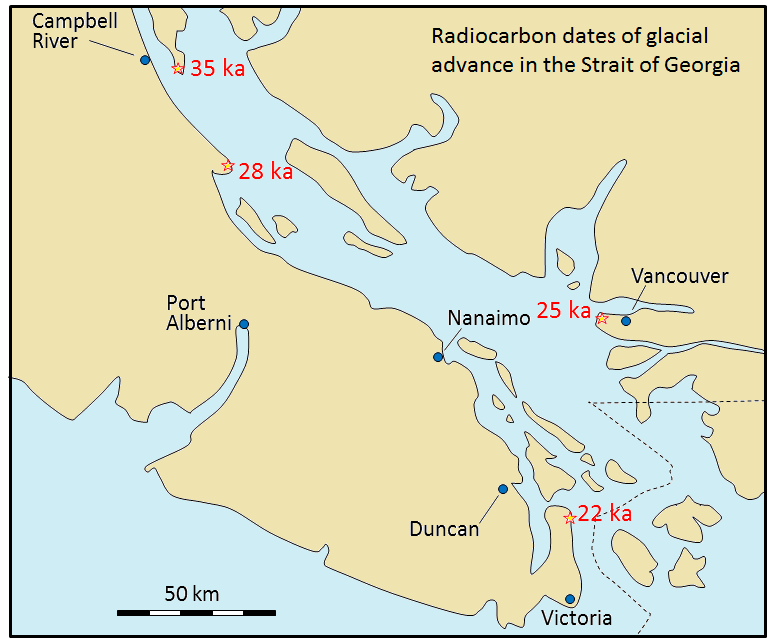
Radiocarbon dating (using 14C) can be applied to many geological materials, including sediment and sedimentary rocks, but only if the materials in question are younger than ~60 ka, and contain organic material. Beyond this time, there is so little 14C left that it cannot be measured accurately, resulting in unreliable age dates. Fragments of wood incorporated into young sediment are good candidates for carbon dating, and this technique has been used widely in studies involving late Pleistocene glaciers and glacial sediments. Figure 19.18 shows radiocarbon dates from wood fragments in glacial sediments have been used to estimate the time of the last glacial advance along the Strait of Georgia.
Putting It Together: Using Multiple Methods to Date a Sedimentary Layer
See your lab results below, then use the following reference materials to answer a few questions.
Note: You might have to fill in some details on your own, because it looks like the lab didn’t complete the reports.
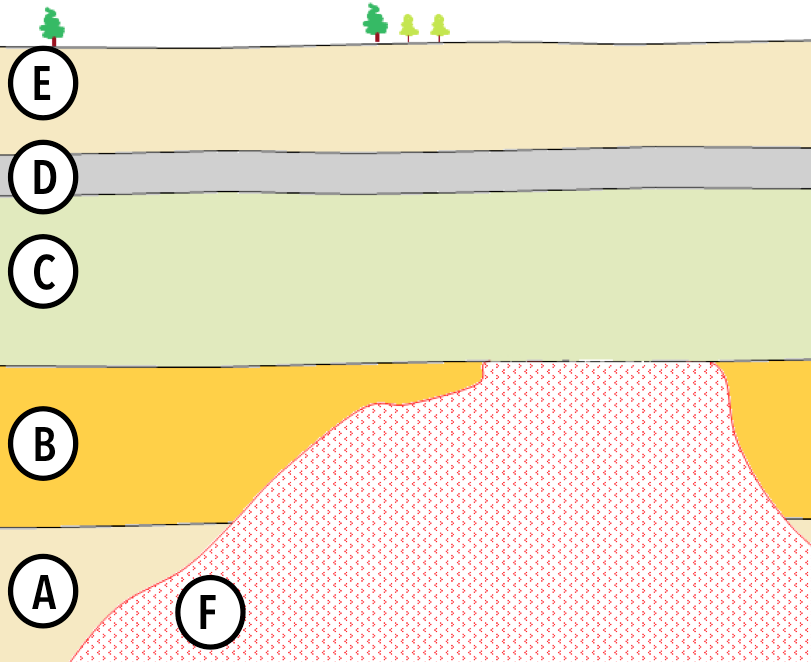
Lab Report 1. Sample: Wood fragment from Bed D. Result: 55% of parent isotope remaining.
Lab Report 2. Sample: Potassium feldspar from Intrusion F. Result: 91% of parent isotope remaining.
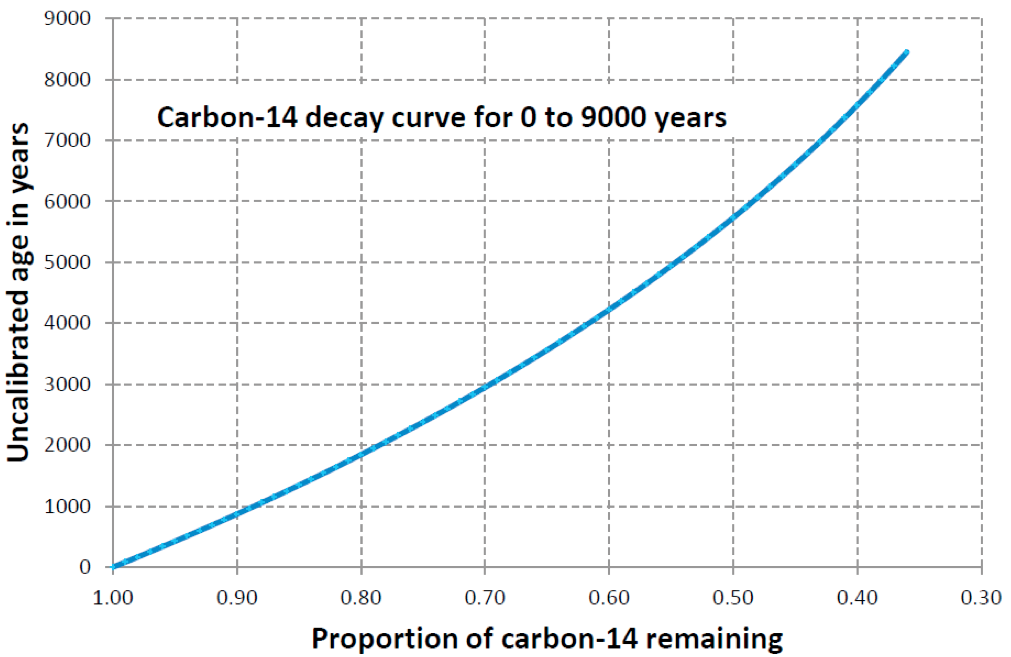
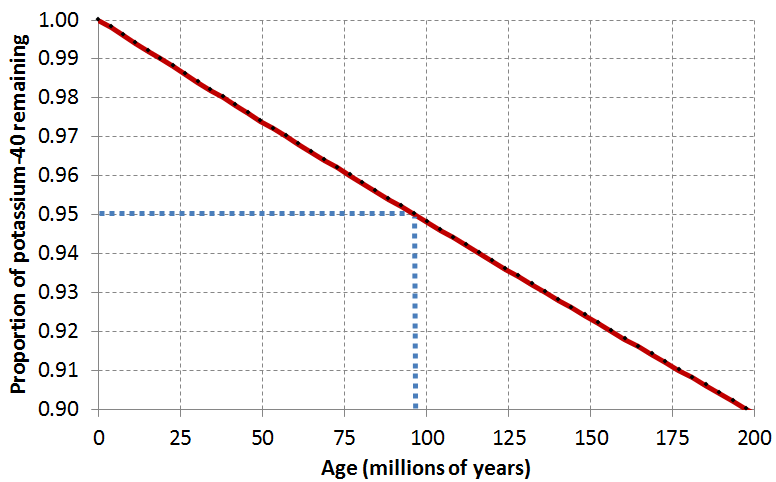
First things first: did you use the right graphs? Fill in the blanks:
The wood sample from Layer D was dated using .
The potassium feldspar crystal was dated using .
Fill-in-the-blank options:
- carbon-14
- potassium-argon
What ages did you get? Fill in the blanks:
The wood samples give an age of years.
The potassium feldspar crystal is Ma old.
To convince the world of your amazing discovery, you need to show that Layer C is very young (geologically speaking, at least).
So far you have a wide range of possible ages, and that’s not getting you any closer to world fame.
But wait! You’ve just noticed that there is a (Hint: Type of unconformity) between Layer C and Intrusion F, which means that there’s also a (Hint: Type of unconformity) between Layers C and B.
You do some research and learn that these are the only features of this kind in your cross-section. This means you can conclude that of the layers you’ve had analyzed, C is much closer in age to (Hint: D or F?) than to (Hint: D or F?).
To check your answers, navigate to the below link to view the interactive version of this activity.
References
Clague, J. (1976). Quadra Sand and its relation to late Wisconsin glaciation of southeast British Columbia. Canadian Journal of Earth Sciences, 13, 803-815.

