2.2 Planet-Forming Materials Come from the Remnants of Exploded Stars
Only four elements account for 95% of Earth’s mass: oxygen (O), magnesium (Mg), silicon (Si), and iron (Fe). Most of the remaining 5% comes from aluminum (Al), calcium (Ca), nickel (Ni), hydrogen (H), and sulphur (S). We know that the big bang made hydrogen and helium, but where did the rest of the elements come from?
The answer is that almost all of the other elements were made by stars. Sometimes stars are said to “burn” their fuel, but burning is not what’s going on within stars. The burning that happens when wood in a campfire is turned to ash and smoke is a chemical reaction: heat causes the atoms that were in the wood and in the surrounding atmosphere to exchange partners. Atoms group in different ways, but the atoms themselves don’t change. What stars do is change the atoms.
Stars Make Small Atoms into Bigger Ones
Heat and pressure within stars cause smaller atoms to smash together and merge into new, larger atoms. For example, when hydrogen atoms smash together and fuse, helium is formed. This process is called nuclear fusion. Large amounts of energy are released when some elements fuse within stars, and that’s what causes stars to shine. Stars can form large quantities of elements as heavy as iron during their normal burning process. Side reactions can form heavier elements in small amounts.
It takes larger stars to make elements as heavy as iron in large quantities. Our sun is an average star. After it uses up its hydrogen fuel to make helium, and some of that helium is fused to make small amounts of other elements, it will be at the end of its life. It will stop making new elements and will cool down and bloat until its middle reaches the orbit of Mars. In contrast, large stars end their lives in spectacular fashion. They explode as supernovae, casting off newly formed atoms into space, and triggering side reactions to make even more heavy atoms. It took many generations of stars creating heavier elements and casting them into space before heavier elements were abundant enough for planets like Earth to form.
Our Planet’s Composition is No Accident.
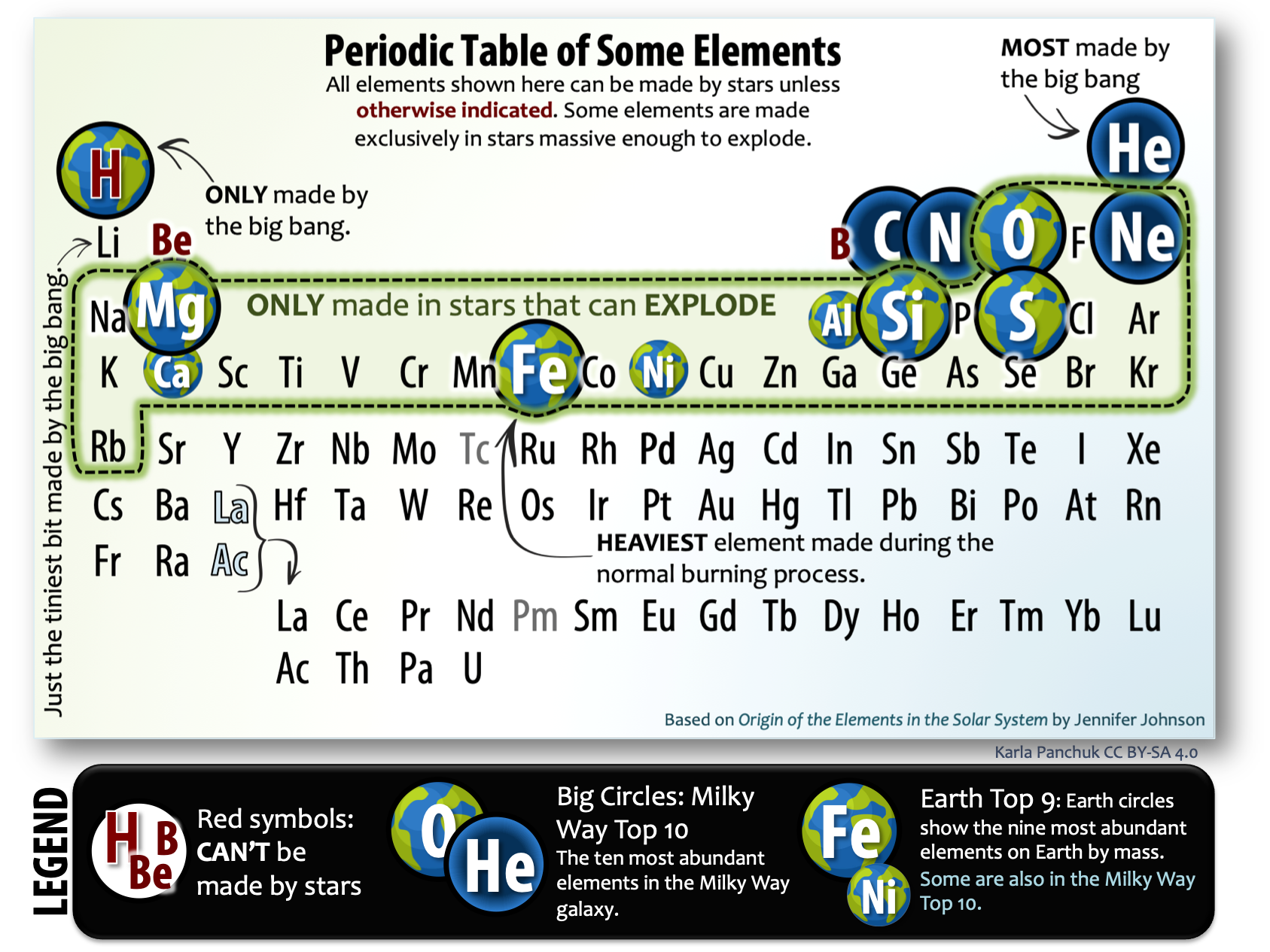
To see the importance of element-forming processes for our planet, notice the large circles in the simplified periodic table in Figure 2.5. The big circles mark the ten most abundant elements in our Milky Way galaxy. Aside from hydrogen and some of the helium, the abundance of those elements is entirely controlled by star processes. Now find the Earth circles. These are the nine most abundant elements we listed earlier. Notice how many of the Earth circles are also big circles. Furthermore, notice how many of the Earth circles fall in the set of elements that require very massive stars to exist and explode.
Do you know your chemical symbols?
References
Johnson, J. (2017, January 9). Origin of the elements in the solar system. Science Blog from the Sloan Digital Sky Surveys. https://blog.sdss.org/2017/01/09/origin-of-the-elements-in-the-solar-system/

