Chapter 7. Parenteral Medication Administration
7.6 Administering Intermittent Intravenous Medication (Secondary Medication) and Continuous IV Infusions
Intravenous intermittent infusion is an infusion of a volume of fluid/medication over a set period of time at prescribed intervals and then stopped until the next dose is required. An intermittent IV medication may be called a piggyback medication, a secondary medication, or a mini bag medication (see Figure 7.16). Intravenous medications may be given in small volumes of sterile IV solution (25 to 250 ml) and infused over a desired amount of time (given for 30 minutes every 4 hours) or as a single dose. Many medications must be given slowly to prevent harm to the patient, and this method of administration reduces the risk of rapid infusion. A piggyback medication is given through an established IV line that is kept patent by a continuous IV solution or by flushing a short venous access device (saline lock). Always check the Parenteral Drug Therapy Manual PDTM to ensure the correct guidelines are followed for each specific medication given in IV solution. The PDTM provides guidelines on how to mix the IV medication, the amount and type of solution, and the rate of infusion (Perry et al., 2014).
An intermittent medication may be administered by gravity or on an electronic infusion device (EID), also known as an infusion (IV) pump. Many piggyback IV medications must be on an IV pump, which requires programming and specialized training to prevent medication errors. The IV infusion pumps provide hard- and soft-dose limits and safety practice guidelines to aid in safe medication administration (Lynn, 2011). IV medications may also be given by gravity infusion, in which case the health care provider must calculate the infusion rate for drops per minute. The best practice for piggyback infusions is to use an IV infusion pump.
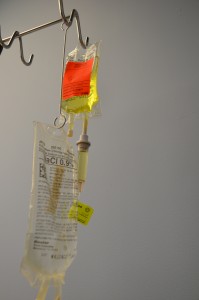
At times, a volume-controlled (intermittent infusion) set may be used to deliver medication for children, older adults, or critically ill patients where fluid volume is a concern. A volume-controlled intermittent set is a small device attached below the primary infusion to regulate the mini bag. The medication is added to a small amount of IV solution and administered through an IV line (Lynn, 2011).
Intravenous medications are always prepared using the seven rights x 3 as per agency policy. Because of the many high-risk events associated with intravenous medications, additional guidelines are required. A PDTM or monograph provides this additional information, which includes the generic name, brand name, classification of the drug, and a chart defining which parenteral route may be utilized. Some medications may only be given via a piggyback method or large-volume IV solutions, and some medications may be given diluted over 1 to 2 minutes. In addition, information on indications, contraindications, dosage (age dependent), administration/dilution guidelines, adverse effects, clinical indications (e.g., specialized monitoring required, must be on an IV pump), and compatibility and incompatibility in relation to reconstitution and primary IV solution are specified (Alberta Health Services, 2009).
The Institute for Safe Medication Practices (2014) has created a list of high-alert medications that bear the heightened risk of significant harm when they are used in error. Specific safeguards for these medications can be found in the PDTM. It is vital to understand which medications are considered high risk prior to administration. A link to the list of high-alert medications can be found under Suggested Online Resources at the end of this chapter. In addition to the seven rights x 3 for medication preparation, Table 7.10 summarizes what to review in the PDTM when preparing and administering an intravenous medication. The acronym RED CARS can be used as a reminder.
|
Mnemonic |
Additional Information |
||
| R | Rate: What is the rate of injection? | ||
| E | Equipment: Equipment may include a syringe, filter needle (vial), or non-filter needle (ampule), label for the syringe, alcohol swab, solution to prepare the medications, PDTM, MAR, non-sterile gloves, normal saline flushes, and a watch with a second hand. | ||
| D | Dilution: How much solution is required to dilute the medication, and what type of solution (normal saline or dextrose)? Some intravenous medications must be administered in a piggyback or mini bag, and some may be given diluted directly into the vein through an existing IV line. | ||
| C | Compatibility: Is the medication compatible with the primary IV solution and additives? NEVER inject intravenous medications into blood or blood products, or IV continuous infusions such as heparin IV or insulin IV. | ||
| A | Allergies: What are the patient’s allergies and types of reactions? | ||
| R | Reconstitution: If the medication requires reconstitution, follow directions for adding the correct amount of dilution and type of dilution. Use the information to accurately achieve the correct concentration of the medication. | ||
| S | Stability: How long is the medication stable at room temperature or when reconstituted? | ||
| Data source: BCIT, 2015 | |||
Special considerations when preparing IV intermittent medications:
- Indications and contraindications exist with most IV medications, such as “use cautiously in patients with a penicillin allergy” or “do not administer in patients with a low potassium level” or “monitor for side effects in patients with liver or kidney failure.” Clinical indications may include “must be on a cardiac monitor” or “only to be administered in a specialized area such as ICU and CCU.”
- Always prepare the medications using the seven rights x 3 when the medication is pulled from storage, poured, and put away.
- Many IV intermittent medications come prepared from the pharmacy and still require a complete check (seven rights x 3) prior to administration.
- The IV solution bag must be labelled at the medication cart with the patient’s name, date, time, medication, dose concentration, and your initials.
- Calculate IV rate (gravity or IV pump) before going to the bedside.
Using sterile technique, prepare the intravenous medication as per agency policy, using the PDTM and the seven rights x 3. Many piggyback medications come prepared from the pharmacy and still require a complete check (SEVEN rights x 3) prior to administration. Checklist 63 lists the steps to administering an intermittent IV medication by gravity or an IV infusion pump.
Disclaimer: Always review and follow your hospital policy regarding this specific skill. |
|||
Safety Considerations:
|
|||
Steps |
Additional Information |
||
| 1. Prepare one medication for one patient at the correct time as per agency policy. Always check the physician orders, PDTM, and MAR.
Mathematical calculations may be required to determine the correct dose to prepare. |
Always apply the SEVEN rights of medication administration.
Review the agency policy and the PDTM. If a medication is a stat, first-time, loading, or one-time dose, be extra diligent in reviewing the PDTM. Memory slips are a common source of error with medication administration. Complete all assessments (vital signs) and check laboratory values that may influence the medication administration. If piggyback (secondary) medication is made up by the health care provider, ensure the medication label on the mini bag includes the patient name, date, time, medication added, dose and concentration, expiry time, and your initials. Some health agencies require a second independent check with high-alert medications. Always follow agency policy. |
||
| 2. Perform hand hygiene and bring medication and MAR to bedside. Create privacy if possible. | Additional equipment required includes secondary tubing, a metal or plastic extension hanger, an alcohol swab, and a timer with a second hand.
Creating privacy provides comfort to patient. |
||
| 3. Compare the MAR with the patient’s wristband, and use two patient identifiers (name and birth date), according to agency policy, to confirm patient ID. | This ensures you have the correct patient and complies with agency standard for patient identification.
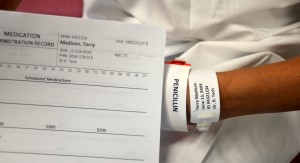 |
||
| 4. Ask about allergies. | This ensures allergy status is correct on the MAR and patient’s allergy band. | ||
| 5. Discuss purpose, action, and possible side effects of the medication. Provide patient an opportunity to ask questions. Encourage patient to report discomfort at the IV site (pain, swelling, or burning). | Keeping patient informed of what is being administered helps decrease anxiety. | ||
| 6. Perform hand hygiene. | Hand hygiene prevents the transmission of microorganisms. | ||
| 7. Assess IV site. Select upper port on the IV tubing. | IV medications may require assessment of vital signs and lab values prior to administration. | ||
| 8. Complete necessary assessments as required. Assess IV site and flush for patency. | Ensure IV site is free from redness, swelling, and pain prior to administering the medication.
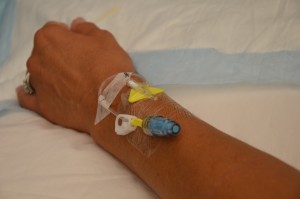 |
||
| 9. Prime secondary tubing. | Remove secondary tubing from packaging and close the clamp. Hang the medication IV bag on the IV pole and remove the sterile blue protective cap.
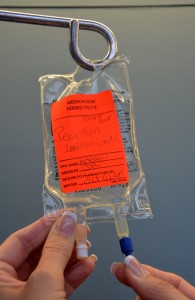  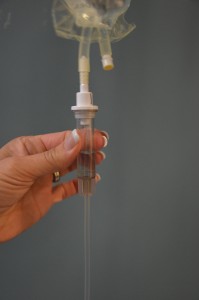 Slowly open the clamp and prime the tubing. 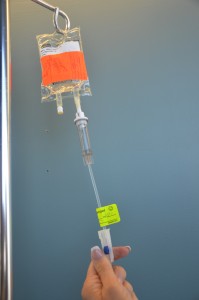 Clean the upper injection port on the primary IV tubing with an alcohol swab for 15 seconds using a circular motion. 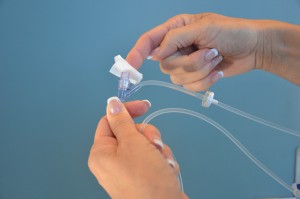 Allow to dry. Remove the cap on the distal end of the secondary tubing and carefully insert the upper injection port. 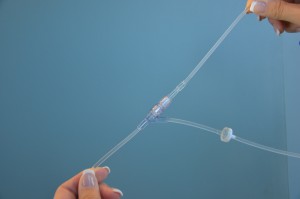 Label the secondary tubing with date and time. 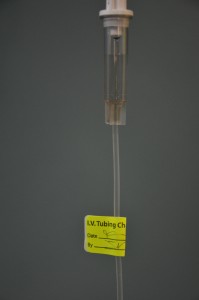 |
||
| 10. Lower the primary IV solution bag using the extension hook. | Ensure piggyback mini bag is hung above the primary IV solution bag. Position of the IV solutions influences the flow of the IV fluid into the patient. The setup is the same if the medication is given by gravity or through an IV infusion pump. Always follow manufacturer’s directions for infusion pumps.
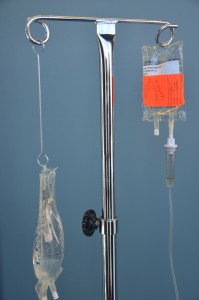 |
||
| 11. Ensure clamp on secondary tubing is open. | This prevents the patient from missing a dose of medication.
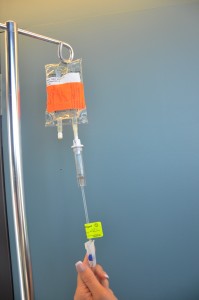 |
||
| 12a. If using gravity infusion, use the roller clamp on the primary set to regulate the rate. The rate will need to be calculated for gtts/mins.
12b. If using an IV infusion pump, set the rate according to the PDTM. Most infusion pumps automatically restart the primary infusion at the previously established rate. |
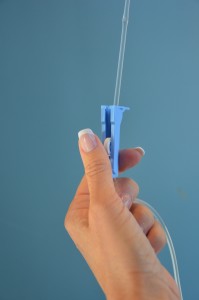 If the medication is administered via gravity, remember to return to the patient and readjust the rate for the primary IV infusion. The primary IV solution will resume infusing at the rate of the secondary infusion, which could lead to rapid infusion of the primary solution. If medication is being given for the first time, stay with the patient for the first 5 minutes to monitor for any potential adverse effects. Encourage patient to notify the health care provider if IV site becomes red, painful, or swollen, or if patient notices any adverse effects from the medication. |
||
| 13. Leave IV piggyback mini bag and tubing in place for future drug administration. Check agency policy to verify if this practice is acceptable. | Repeated changes in IV tubing increase risk for infection transmission. Secondary IV tubing should be changed as per agency policy. | ||
| 14. Perform hand hygiene. | Hand hygiene reduces the transmission of microorganisms.
 |
||
| 15. Document administration of the IV piggyback on MAR, the I/O sheet, and as per agency policy. | Document time, therapeutic effect, and any adverse reactions.
Prompt documentation avoids the possibility of accidentally repeating the administration of the drug. If the drug was omitted or refused, record this appropriately and notify the primary health care provider. |
||
| Data source: Berman & Snyder, 2016; Lynn, 2011; Perry et al., 2014; WHO, 2012 | |||
Checklist 64 lists the steps to administer an intermittent IV medication using an existing secondary line, by gravity or an IV infusion pump.
Disclaimer: Always review and follow your hospital policy regarding this specific skill. |
|||
Safety Considerations:
|
|||
Steps |
Additional Information |
||
| 1. Prepare one medication for one patient at the correct time as per agency policy. Always check the physician’s order, PDTM, and MAR.
Mathematical calculations may be required to determine the correct dose to prepare. |
Always apply the SEVEN rights of medication administration.
Review the agency policy and the PDTM. If a medication is a stat, first-time, loading, or one-time dose, be extra diligent in reviewing the PDTM. Memory slips are a common source of error with medication administration. Complete all assessments and laboratory values that may influence the medication administration. If piggyback (secondary) medication is made up by the health care provider, ensure the medication label on the mini bag includes the patient name, date, time, medication added, dose and concentration, expiry time, and your initials. Some health agencies require a second independent check with high-alert medications. Always follow agency policy. |
||
| 2. Bring medication and MAR to bedside. Create privacy if possible. | Additional equipment required includes secondary tubing, a metal or plastic extension hanger, an alcohol swab, and a timer with a second hand.
Creating privacy provides comfort to patient. |
||
| 3. Compare the MAR printout with the patient’s wristband, and use two patient identifiers (name and birth date), according to agency policy, to confirm patient ID. | This ensures you have the correct patient and complies with agency standard for patient identification.
 |
||
| 4. Ask about allergies. | This ensures allergy status is correct on the MAR and the patient’s allergy band. | ||
| 5. Discuss purpose, action, and possible side effects of the medication. Provide patient an opportunity to ask questions. Encourage patient to report discomfort at the IV site (pain, swelling, or burning). | Keeping patient informed of what is being administered helps decrease anxiety. | ||
| 6. Perform hand hygiene. | Hand hygiene prevents the transmission of microorganisms.
 |
||
| 7. Complete necessary assessments as required. Assess IV site for patency. | IV medications may require assessment of vital signs and lab values prior to administration.
IV site must be patent prior to use. |
||
| 8. Prime the secondary IV line by “back filling” using the empty IV mini bag attached to the secondary IV line. | Check expiration date on secondary IV tubing.
Open the clamp on the secondary IV line and lower the mini bag below the primary IV line. This will cause IV solution from the primary IV bag to enter the old mini bag and clear out the secondary IV line. Allow approximately 25 ml of IV solution to enter the used mini bag. 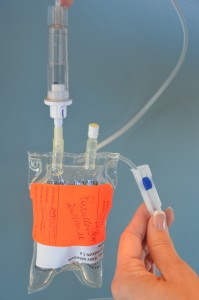 Once the secondary IV line is cleared, close the clamp on the secondary IV line, and ensure the drip chamber is 1/2 full. Remove the old mini bag from the secondary IV tubing and place on the bedside table. Carefully remove sterile blue cover on new medication bag, and insert the spike of the secondary IV tubing into the new IV bag, being careful to avoid accidental contamination. 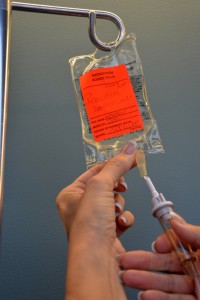 Open clamp on the secondary IV tubing.  |
||
| 9. Ensure piggyback mini bag is hung above the primary IV solution bag. | Position of the IV solutions influences the flow of the IV fluid into the patient. The setup is the same if the medication is given by gravity or through an IV infusion pump. Always follow manufacturer’s directions for infusion pumps.
 |
||
| 10. Ensure clamp on secondary tubing is open. | This prevents the patient from missing a dose of medication.
 |
||
| 11a. If using gravity infusion, use the roller clamp on the primary set to regulate the rate. The rate will be calculated for gtts/mins.
11b. If using an IV infusion pump, set the rate according to the PDTM. Most infusion pumps automatically restart the primary infusion at the previously established rate. |
 If administering IV medication by gravity, remember to return to the patient and readjust the rate for the primary IV infusion. The primary IV solution will resume infusing at the rate of the secondary infusion, which could lead to rapid infusion of the primary solution. If medication is being given for the first time, stay with the patient for the first 5 minutes to monitor for any potential adverse effects. Encourage patient to notify the health care provider if IV site becomes red, painful, or swollen, or if patient notices any adverse effects from the medication. |
||
| 12. Leave IV mini bag and tubing in place for future drug administration. Check agency policy to verify if this practice is acceptable. | Repeated changes to IV tubing increase risk for infection transmission. Secondary IV tubing should be changed as per agency policy. | ||
| 13. Perform hand hygiene. | Hand hygiene reduces the transmission of microorganisms.
 |
||
| 14. Document administration of the IV piggyback on MAR, and as per agency policy. | Document time, therapeutic effect, and any adverse reactions
Prompt documentation avoids the possibility of accidentally repeating the administration of the drug. If the drug was omitted or refused, record this appropriately and notify the primary health care provider. |
||
| Data source: Clayton et al., 2010; Lynn, 2011; Perry et al., 2014; WHO, 2012 | |||
Continuous Intravenous (Medication) Infusion
A continuous intravenous infusion is the infusion of a parenteral drug over several hours (continuous drip) to days. It involves adding medication to sterile IV solution (100 to 1,000 ml bag), and then hanging the IV solution as a primary infusion. A continuous drip must be ordered by the physician and listed in the PDTM as a medication to be given by IV continuous infusion. Most IV continuous infusions are given for a short duration. Examples of continuous IV infusion medications include heparin, insulin R, and pantaprazole. Continuous intravenous infusions may come pre-made from the pharmacy, and are labelled with the patient name; IV solution; volume, amount, and concentration of medication; initials of the RN; and date and time prepared (Alberta Health Services, 2009). Always refer to the PDTM for guidelines on how to administer, regulate, and titrate continuous infusions.
An electronic infusion device (EID) must be used to infuse continuous IV medications. Assessments and lab values must be monitored following the PDTM guidelines. A health care provider must assess the continuous medication for the dose, rate, and patency of the IV site, and assess the patient for therapeutic and adverse reactions to the medication. The Institute for Safe Medication Practices (ISMP) (2013) recommends that all high-alert medications be independently double-checked to detect potential harmful errors before they reach the patient. Independent double checks have been shown to detect up to 95% of errors (ISMP Canada, 2013).
Critical Thinking Exercises
- Can the same secondary IV tubing be used more than once? Explain your answer.
- What is the purpose of hanging the piggyback IV medication higher than the primary IV solution?

