Chapter 8. Intravenous Therapy
8.7 Transfusion of Blood and Blood Products
All health care practitioners who administer blood or blood products must complete specific training for safe transfusion practices and be competent in the transfusion administration process. Always refer to your agency policy for guidelines for preparing, initiating, and monitoring blood and blood product transfusions. These guidelines apply to adult patients only.
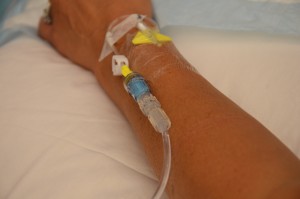
The transfusion of blood or blood products (see Figure 8.8) is the administration of whole blood, its components, or plasma-derived products. The primary indication for a red blood cell (RBC) transfusion is to improve the oxygen-carrying capacity of the blood (Canadian Blood Services, 2013). A health care provider order is required for the transfusion of blood or blood products. RBC transfusions are indicated in patients with anemia who have evidence of impaired oxygen delivery. For example, individuals with acute blood loss, chronic anemia and cardiopulmonary compromise, or disease or medication effects associated with bone marrow suppression may be candidates for RBC transfusion. In patients with acute blood loss, volume replacement is often more critical than the composition of the replacing fluids (Canadian Blood Services, 2013). Transfusions can restore blood volume, restore oxygen-carrying capacity of blood with red blood cells, and provide platelets and clotting factors. The most common type of blood transfusion is blood that is donated by another person (allogeneic). Autologous transfusion is the transfusion of one’s own blood (Perry et al., 2014).
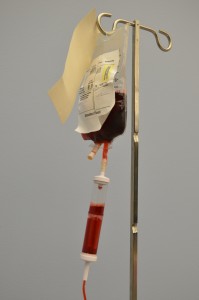
Transfusion therapy is considered safe, and stringent precautions are followed in the collection, processing, and administration of blood and blood components. However, transfusions still carry risks such as incompatibility, human error, and disease transmission, and blood transfusion must be taken seriously at all times. Incompatibility can be decreased by using irradiated red blood cells or leukocyte-reduced blood. The majority of blood transfusion complications are a result of human error (Perry et al., 2014).
Compatibility testing is vital for all recipients of blood or blood products. Recipients must be transfused with an ABO group specific to their own blood type or ABO group-compatible. There are three types of blood typing systems: ABO, Rh, and human leukocyte antigen (HLA). For more information on these, refer to the online resources at the end of this chapter. It is vital to understand what types of blood groups are compatible for transfusions (Canadian Blood Services, 2013).
When administering blood and blood products, it is important to know the patient’s values and beliefs regarding blood products. Some groups of individuals, mainly Jehovah Witnesses, will refuse blood transfusions or blood products based on religious beliefs. These individuals will refuse transfusion of whole blood and primary blood components but may accept transfusion of derivatives of primary blood components such as albumins solutions, clotting factors and immunoglobulins. Always assess each individual preference to establish if a blood component is an acceptable treatment to manage their illness or condition (Canadian Blood Services, 2007).
When managing blood transfusions, it is important to prevent complications from occurring and to identify issues promptly to manage reactions effectively. Transfusion reactions (mild to life-threatening) may occur despite all safety measures taken. All transfusion reactions and transfusion errors must be reported to the hospital’s transfusion services (blood bank). It is imperative to know what signs and symptoms to look for, and to educate your patient on what to report and when to report potential transfusion reactions. Mild to severe reactions may include (Canadian Blood Services, 2011):
- Temperature ≥ 38.0 C or change of 1°C from pretransfusion value within 15 minutes after initiation of transfusion
- Acute or delayed hemolytic transfusion reaction
- Hypotension/shock
- Rigors
- Anxiety
- Back or chest pain
- Nausea/vomiting
- Shortness of breath (dyspnea)
- Hemoglobinuria
- Bleeding/pain at IV site
- Tachycardia/arrhythmia
- Generalized flushing
- Rash ≥ 25% of body
- Urticaria and other anaphylaxis reactions
- Hemolysis after transfusion
- Cytopenias after transfusion
- Virus, parasite, and prion infections
- Non-immunological reactions including infection
- Circulatory overload
- Hypothermia
For more information on types of reactions, signs and symptoms, and treatments, review the article adverse events related to blood transfusions, or see the online resources at the end of this chapter. If patient has a blood transfusion reaction, always follow agency policy to manage mild to severe blood reactions. In general, if a reaction occurs, follow the steps outlined in Checklist 73.
In preparation for a blood or blood product transfusion (Alberta Health Services, 2015a, 2015b; Perry et al., 2014; Vancouver Coastal Health, 2008), the steps listed in Checklist 74 must be completed. These steps must be completed before obtaining the blood or blood product from the blood bank.
Checklist 75 provides steps to administering blood and blood products safely in the acute care setting.
Disclaimer: Always review and follow your hospital policy regarding this specific skill. |
||||
Safety considerations:
|
||||
Steps |
Additional Information |
|||
| 1. Verify physician orders and all preparation steps as listed in Checklist 74. | ||||
| 2. Assess or initiate venous access. | Appropriate needle gauge is based on clinical status of patient, urgency of transfusion, and venous access:
Transfusion set must be Luer-locked to a 2.0 ml maximum extension tubing, either directly to cannula or through a Max Plus positive pressure cap.  |
|||
| 3. Initiate primary infusion at TKVO. | Prime an IV line following Checklist 66.
Refer to blood product fact sheets for all other products.  |
|||
| 4. Complete and document cardiovascular assessments and initial vital signs. | Document any clinical sign or symptom that may be confused with a transfusion reaction (e.g., existing fever). | |||
| 5. Obtain products from the transfusion areas within 30 minutes of planned transfusion. | Plan for pickup or delivery of blood and blood products. Do not request blood or blood products if Steps 1 to 4 are not complete. | |||
| 6. Complete visual inspection of product. | Assess blood bag for any signs of leaks or contamination, such as clumping, clots, gas bubbles, or a purplish discoloration. Return to blood bank if blood bag contains any of the above signs.
 |
|||
| 7. Initial verification:
a. Compare the transfusion medical services (TMS) documentation with the patient record to verify:
b. Compare the TMS documentation with the product label attached to the product tab and verify:
|
All verification numbers/information must match exactly.
Must be completed by two trained staff members competent in blood transfusion administration process as set out by the agency. Confirm the patient blood type and Rh are compatible with the donor blood type and Rh. If there are any discrepancies, stop the process and contact the TMS for resolution and direction. Do not proceed. Ensure the blood product matches the physician’s orders (red blood cells or platelets).  |
|||
| 8 Administer pre-medications as ordered. | Medications must be administered through an IV infusion set, and the IV site cleared with 0.9% NS. | |||
| 9. Final verification (must be completed by the same two staff members as noted in Step 7).
Compare the patient’s first and last name and unique identifier number using all of the following:
Only after recipient identification and product check is confirmed, invert product 5 to 10 times and insert spike of the blood administration set into the blood product container. |
All verification numbers must match exactly. If there are any discrepancies, stop the process and contact the TMS for resolution and direction. Do not proceed.
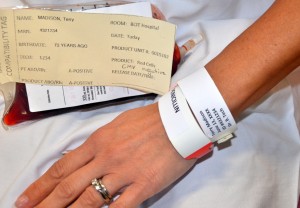 Patients who are alert and oriented should be asked to:
All identifying information attached to the blood bag must remain attached at least until completion of transfusion. |
|||
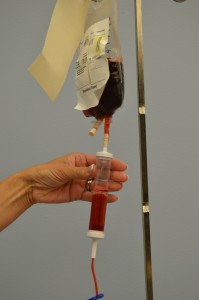
10. Perform hand hygiene. Prime the blood product administration set:
|
Do not remove the product from the presence of the patient; prime at bedside. If product is removed from bedside, the final verification process must be completed again.
 |
|||
11. Initiate transfusion:
|
Adults: Initiate red cells slowly (25 ml in the first 15 minutes). For all other blood transfusions, refer to the blood and product sheet as per your agency policy.
Some agencies use an EID to administer blood transfusions. Always check agency policy prior to transfusion. For each and every unit:
Most transfusion reactions occur within first 15 minutes of a transfusion. Infusing small amounts of blood component initially minimizes volume of blood to which patient is exposed, thereby minimizing severity of reaction. 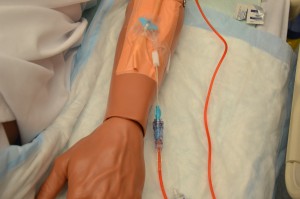 |
|||
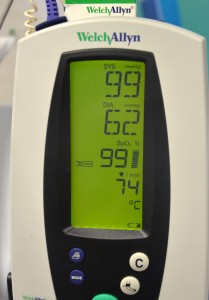
12. Monitor:
|
Vital signs must be monitored:
 |
|||
| 13. In the event of a transfusion reaction, stop the infusion. |
|
|||
| 14. For additional units, repeat steps 6 to 12. | Follow the same process to ensure patient safety. | |||
| 15. Flush administration set with maximum of 50 ml of normal saline and re-establish IV or SL as per physician orders. | Flushing displaces any blood or blood product from the administration set. It is not necessary to flush between units of the same blood product. | |||
| 16. Discard waste in biohazard waste container. | This prevents the spread of biohazard waste. | |||
| 17. Complete all documentation as required by agency. | Documentation may include:
|
|||
| Data source: Alberta Health Services, 2015a, 2015b; Perry et al., 2014; Vancouver Coastal Health, 2008 | ||||
Critical Thinking Exercises
- How many units of blood can be transfused through one blood administration set?
- What are the steps to managing a blood transfusion reaction?